

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089055 (CHEMBL273811 | N*2*-(4-Ethanesulfonylmethyl-phenyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089048 (CHEMBL18163 | N,N-Dimethyl-C-[4-(4-phenylamino-qui...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50089038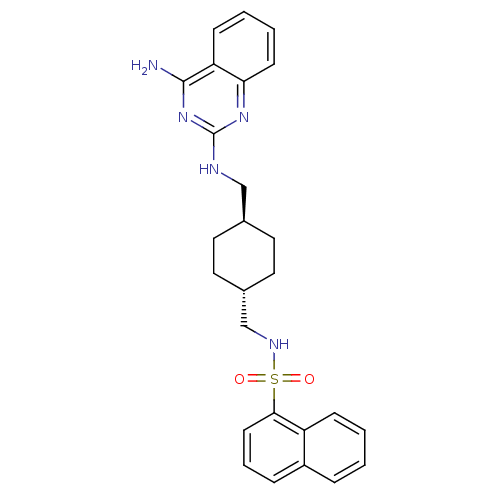 (CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for rat Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089069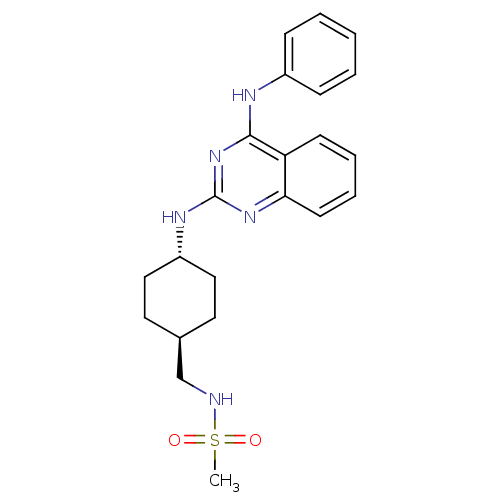 (CHEMBL17632 | N-[4-(4-Phenylamino-quinazolin-2-yla...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089060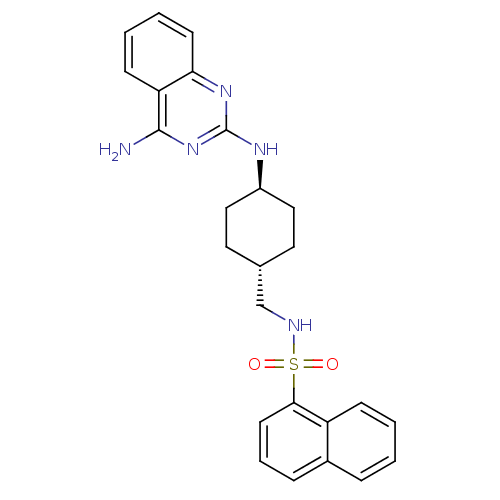 (CHEMBL273597 | Naphthalene-1-sulfonic acid [4-(4-a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089038 (CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its antagonistic activity against Neuropeptide Y receptor Y5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089038 (CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for human Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089036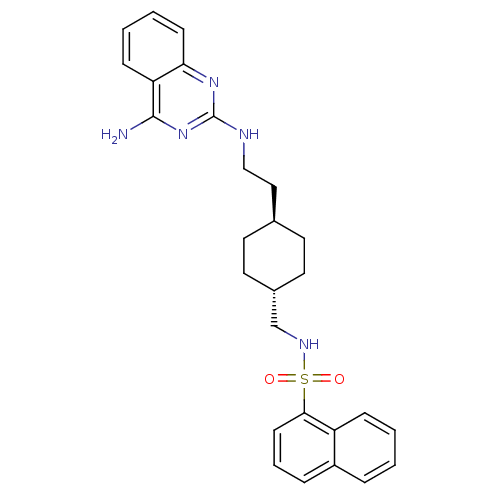 (CHEMBL278881 | Naphthalene-1-sulfonic acid {4-[2-(...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089070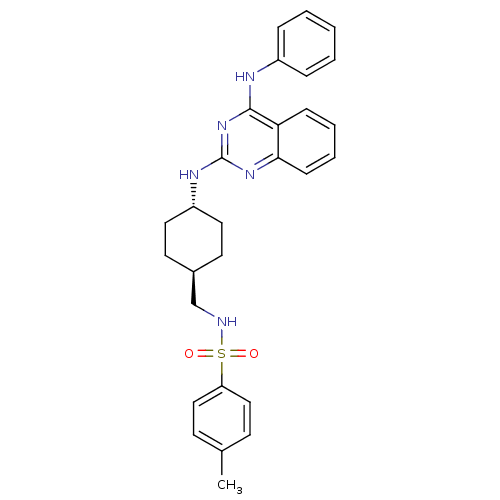 (4-Methyl-N-[4-(4-phenylamino-quinazolin-2-ylamino)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089065 (CHEMBL279408 | N*2*-(4-Diethylamino-phenyl)-N*4*-p...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089063 (Acetic acid 4-(4-phenylamino-quinazolin-2-ylamino)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089066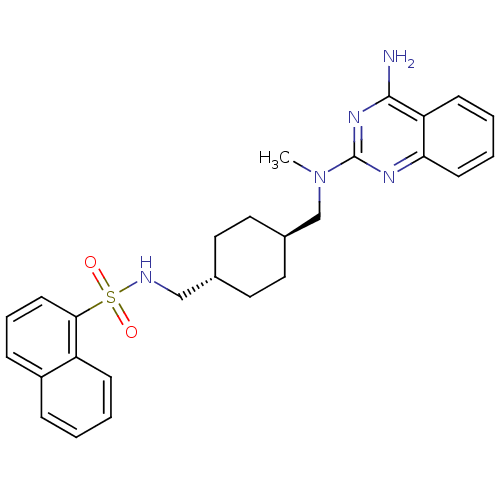 (CHEMBL276768 | Naphthalene-1-sulfonic acid (4-{[(4...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089050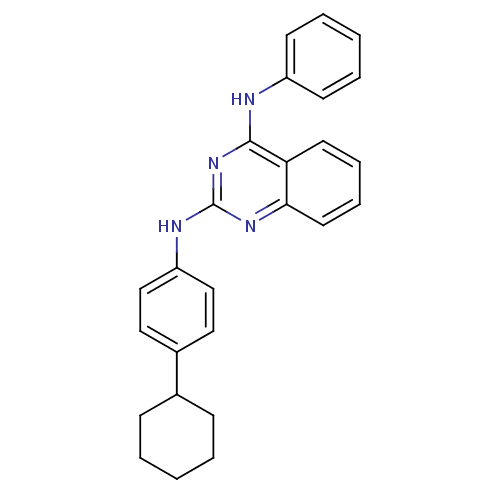 (CHEMBL16936 | N*2*-(4-Cyclohexyl-phenyl)-N*4*-phen...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089058 (CHEMBL276551 | Naphthalene-1-sulfonic acid [6-(4-a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089054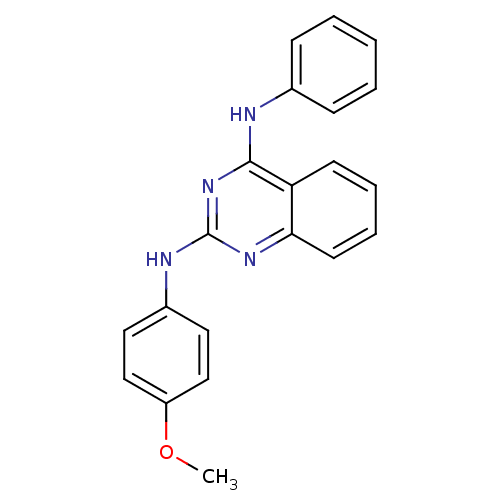 (CHEMBL17352 | N*2*-(4-Methoxy-phenyl)-N*4*-phenyl-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089044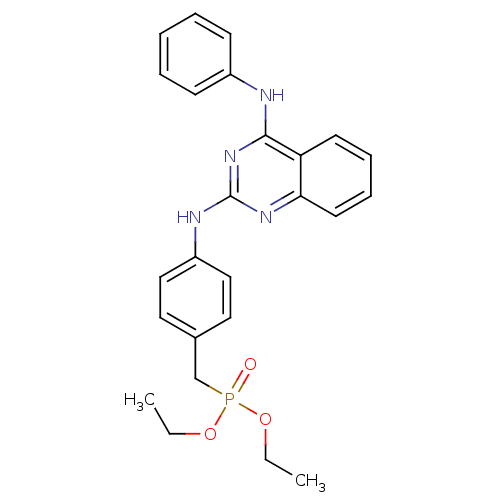 (CHEMBL17686 | [4-(4-Phenylamino-quinazolin-2-ylami...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089033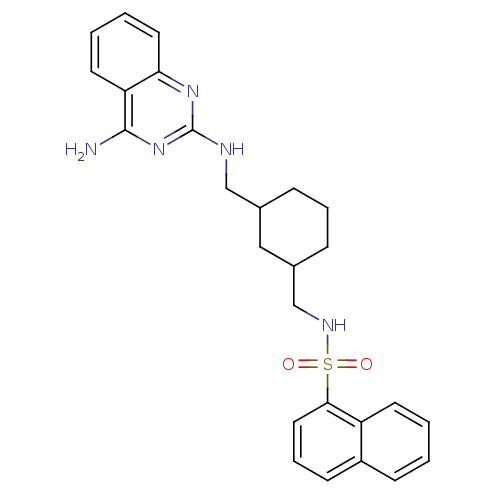 (CHEMBL418359 | Naphthalene-1-sulfonic acid {3-[(4-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089045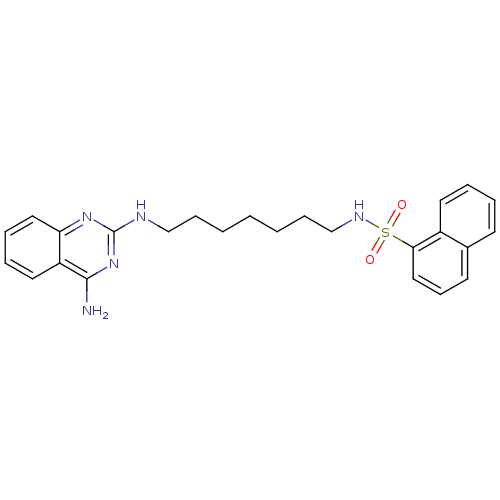 (CHEMBL17196 | Naphthalene-1-sulfonic acid [7-(4-am...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089037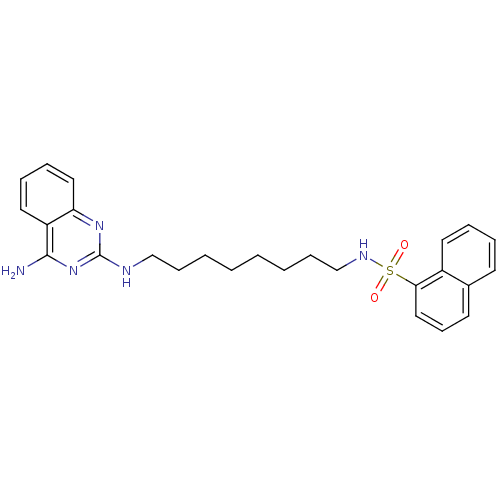 (CHEMBL17701 | Naphthalene-1-sulfonic acid [8-(4-am...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089046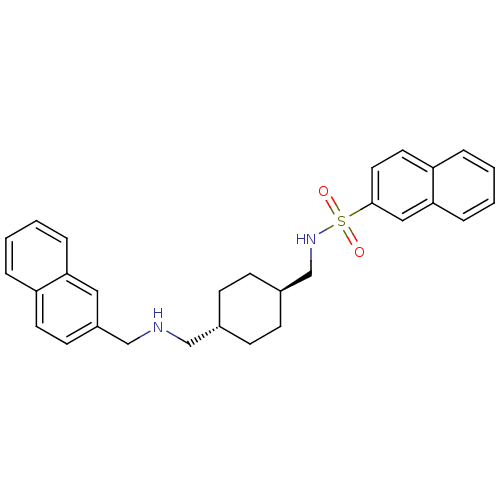 (CHEMBL17022 | Naphthalene-2-sulfonic acid (4-{[(na...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089047 (CHEMBL17914 | N*2*-Cyclohexyl-N*4*-phenyl-quinazol...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089043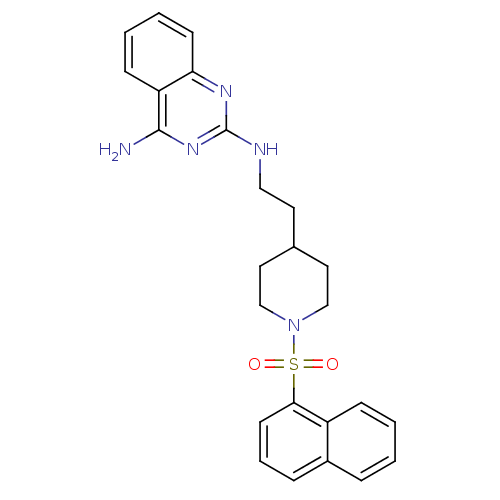 (CHEMBL17417 | N*2*-{2-[1-(Naphthalene-1-sulfonyl)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089032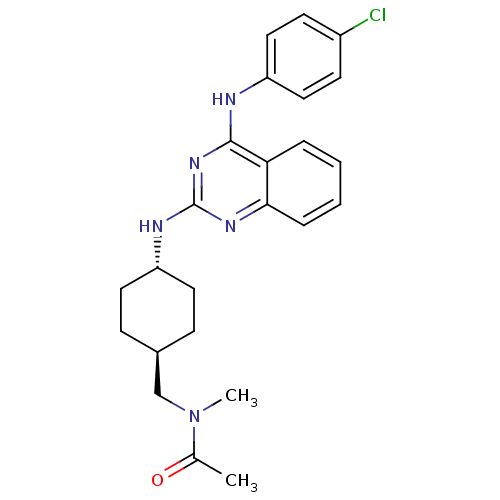 (CHEMBL17395 | N-{4-[4-(4-Chloro-phenylamino)-quina...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089040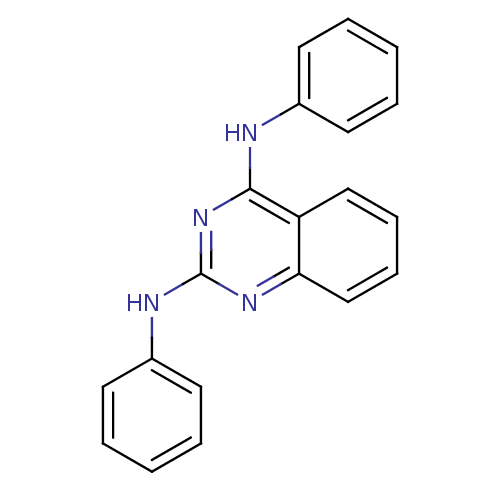 (CHEMBL17483 | N*2*,N*4*-Diphenyl-quinazoline-2,4-d...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089067 (CHEMBL17326 | N*2*-(4-Chloro-phenyl)-N*4*-phenyl-q...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089051 (CHEMBL17911 | Naphthalene-1-sulfonic acid {4-[(4-a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089062 (1'-(4-Amino-6,7-dimethoxy-quinazolin-2-yl)-4-cyclo...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089057 (CHEMBL429053 | N*4*-Phenyl-N*2*-p-tolyl-quinazolin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089061 (CHEMBL17450 | N*2*-(3-Dibutylamino-propyl)-N*4*-(3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089031 (CHEMBL17427 | N-{4-[4-(4-Chloro-phenylamino)-quina...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089034 (CHEMBL17247 | N*2*-(3,4-Dichloro-phenyl)-N*4*-phen...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089053 (CHEMBL278677 | Naphthalene-1-sulfonic acid 4-[(4-a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089056 (CHEMBL279181 | N-{4-[4-(4-Chloro-phenylamino)-quin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089059 (CHEMBL16965 | Naphthalene-1-sulfonic acid 3-[(4-am...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089042 (CHEMBL17398 | N-{4-[4-(4-Chloro-phenylamino)-quina...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089052 (Benzoic acid 4-(4-phenylamino-quinazolin-2-ylamino...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089049 (CHEMBL17454 | N*4*-(4-Chloro-phenyl)-N*2*-(4-methy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089064 (CHEMBL275845 | Naphthalene-1-sulfonic acid (4-{[(4...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50089061 (CHEMBL17450 | N*2*-(3-Dibutylamino-propyl)-N*4*-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 1 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089041 (CHEMBL275610 | [1-(Naphthalene-1-sulfonyl)-piperid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089035 (2-[1-(4-Amino-6,7-dimethoxy-quinazolin-2-yl)-piper...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50089038 (CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for human Neuropeptide Y receptor type 2 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089039 (CHEMBL17441 | N*2*-(4-Amino-butyl)-6-phenyl-quinaz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089068 (CHEMBL277551 | Naphthalene-1-sulfonic acid [(1R,2S...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonistic activity against neuropeptide Y receptor type 5 subtype stably expressed in LM(tk-)cells | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50089038 (CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for human Neuropeptide Y receptor type 4 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50089039 (CHEMBL17441 | N*2*-(4-Amino-butyl)-6-phenyl-quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 1 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50089038 (CGP 71683 | CGP-71683A | CHEMBL17645 | N-{[(1r,4r)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for human Neuropeptide Y receptor type 1 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50089062 (1'-(4-Amino-6,7-dimethoxy-quinazolin-2-yl)-4-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 1 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50089046 (CHEMBL17022 | Naphthalene-2-sulfonic acid (4-{[(na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 1 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50089035 (2-[1-(4-Amino-6,7-dimethoxy-quinazolin-2-yl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Compound was tested for its affinity to bind with Neuropeptide Y receptor type 1 | Bioorg Med Chem Lett 10: 1175-9 (2000) BindingDB Entry DOI: 10.7270/Q2KH0MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||