 Found 74 hits Enz. Inhib. hit(s) with all data for entry = 50018173
Found 74 hits Enz. Inhib. hit(s) with all data for entry = 50018173 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405744
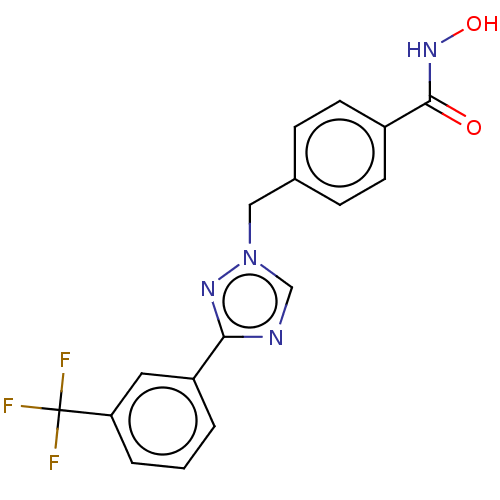
(CHEMBL5274088)Show SMILES COc1ccccc1CNCCCCCCN(C)CCCCCCCCN(C)CCCCCCNCc1ccccc1OC Show InChI InChI=1S/C38H66N4O2/c1-41(31-21-11-7-17-27-39-33-35-23-13-15-25-37(35)43-3)29-19-9-5-6-10-20-30-42(2)32-22-12-8-18-28-40-34-36-24-14-16-26-38(36)44-4/h13-16,23-26,39-40H,5-12,17-22,27-34H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405742
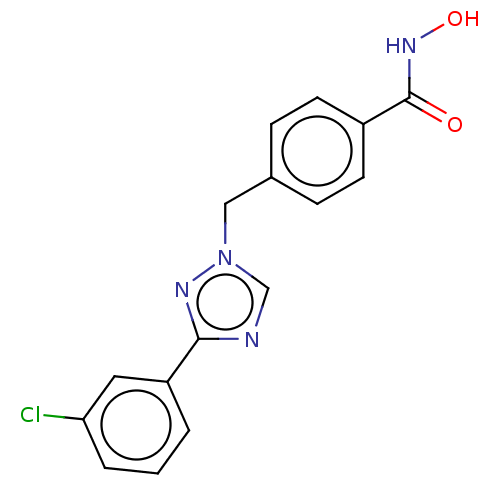
(CHEMBL5271520)Show SMILES COc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(OC)cc2)cc1 Show InChI InChI=1S/C36H62N4O2/c1-41-35-21-17-33(18-22-35)31-39-29-15-9-7-13-27-37-25-11-5-3-4-6-12-26-38-28-14-8-10-16-30-40-32-34-19-23-36(42-2)24-20-34/h17-24,37-40H,3-16,25-32H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405753
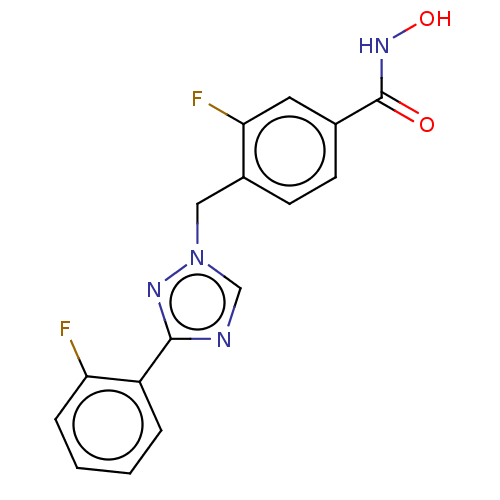
(CHEMBL5274634)Show SMILES Clc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C34H56Cl2N4/c35-33-19-15-31(16-20-33)29-39-27-13-7-5-11-25-37-23-9-3-1-2-4-10-24-38-26-12-6-8-14-28-40-30-32-17-21-34(36)22-18-32/h15-22,37-40H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405753

(CHEMBL5274634)Show SMILES Clc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C34H56Cl2N4/c35-33-19-15-31(16-20-33)29-39-27-13-7-5-11-25-37-23-9-3-1-2-4-10-24-38-26-12-6-8-14-28-40-30-32-17-21-34(36)22-18-32/h15-22,37-40H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405681
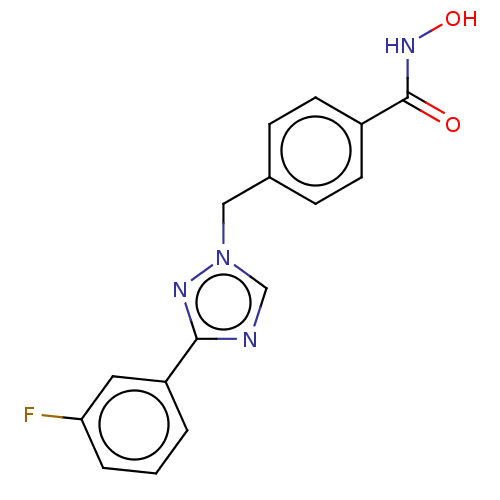
(CHEMBL5271793)Show InChI InChI=1S/C14H14N/c1-15-11-9-14(10-12-15)8-7-13-5-3-2-4-6-13/h2-12H,1H3/q+1/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405679
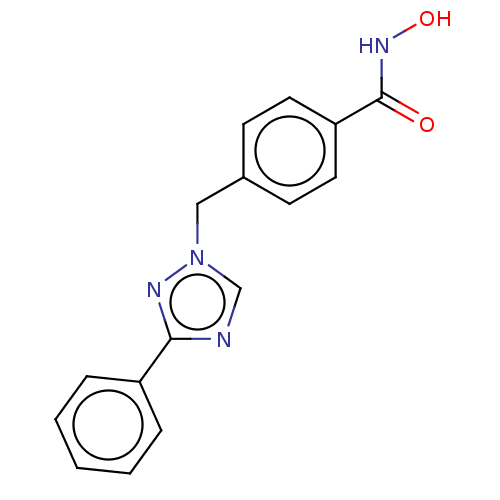
(CHEMBL5266236)Show InChI InChI=1S/C18H21N/c1-19-13-11-15(12-14-19)9-10-17-7-4-6-16-5-2-3-8-18(16)17/h2-10,15H,11-14H2,1H3/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
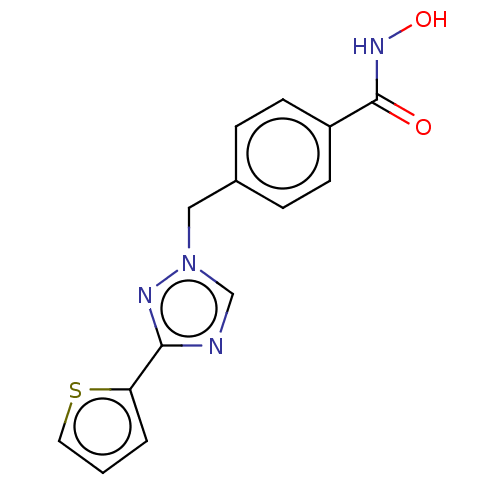
(Homo sapiens (Human)) | BDBM50405750
(CHEMBL5291256)Show InChI InChI=1S/C30H54N4O2/c1(3-9-19-31-21-11-5-7-13-23-33-27-29-17-15-25-35-29)2-4-10-20-32-22-12-6-8-14-24-34-28-30-18-16-26-36-30/h15-18,25-26,31-34H,1-14,19-24,27-28H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
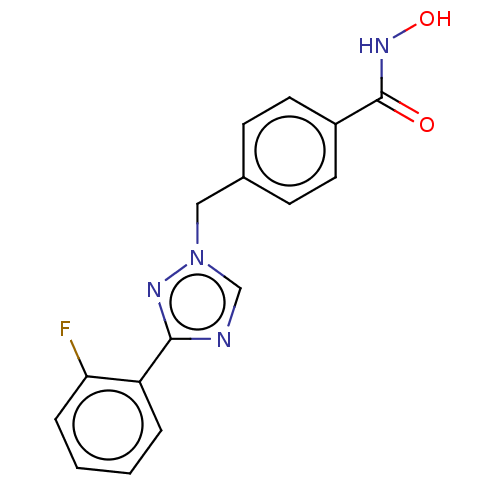
(Homo sapiens (Human)) | BDBM50405680
(CHEMBL5268616)Show InChI InChI=1S/C18H16N/c1-19-14-5-4-10-17(19)13-12-16-9-6-8-15-7-2-3-11-18(15)16/h2-14H,1H3/q+1/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
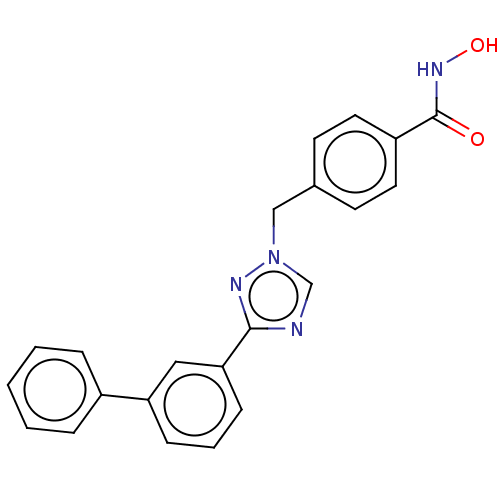
(Homo sapiens (Human)) | BDBM50405748
(CHEMBL5279952)Show SMILES COc1ccccc1CNCCCCCCCNCCCCCCCCNCCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C38H66N4O2/c1-43-37-25-15-13-23-35(37)33-41-31-21-11-5-9-19-29-39-27-17-7-3-4-8-18-28-40-30-20-10-6-12-22-32-42-34-36-24-14-16-26-38(36)44-2/h13-16,23-26,39-42H,3-12,17-22,27-34H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405746
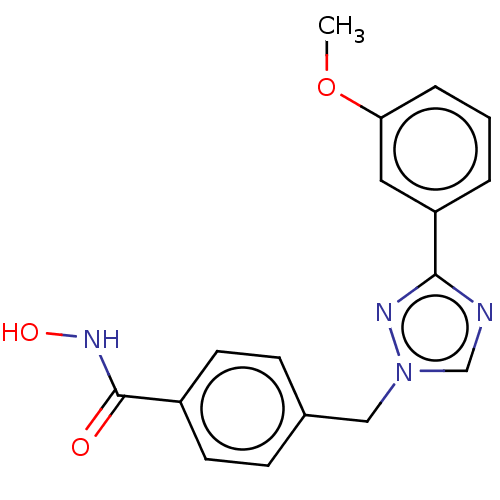
(CHEMBL5286013)Show SMILES [O-][N+](=O)c1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C34H56N6O4/c41-39(42)33-19-15-31(16-20-33)29-37-27-13-7-5-11-25-35-23-9-3-1-2-4-10-24-36-26-12-6-8-14-28-38-30-32-17-21-34(22-18-32)40(43)44/h15-22,35-38H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405747

(CHEMBL5267739)Show SMILES Cc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(C)cc2)cc1 Show InChI InChI=1S/C36H62N4/c1-33-17-21-35(22-18-33)31-39-29-15-9-7-13-27-37-25-11-5-3-4-6-12-26-38-28-14-8-10-16-30-40-32-36-23-19-34(2)20-24-36/h17-24,37-40H,3-16,25-32H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405757
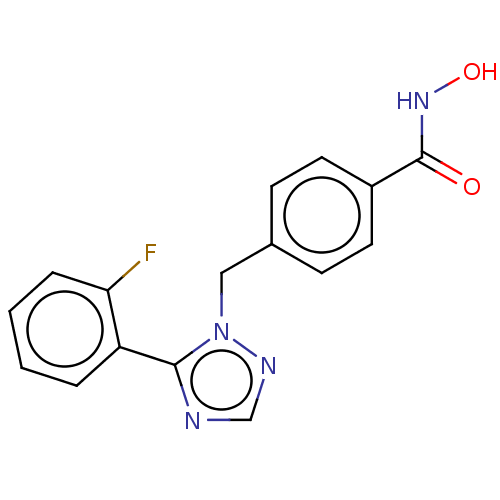
(CHEMBL5271343)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCCCCCCNCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C41H72N4O2/c1-46-40-28-18-16-26-38(40)36-44-34-24-14-12-22-32-42-30-20-10-8-6-4-3-5-7-9-11-21-31-43-33-23-13-15-25-35-45-37-39-27-17-19-29-41(39)47-2/h16-19,26-29,42-45H,3-15,20-25,30-37H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405751

(CHEMBL5281142)Show SMILES Clc1ccccc1CNCCCCCCNCCCCCCCCNCCCCCCNCc1ccccc1Cl Show InChI InChI=1S/C34H56Cl2N4/c35-33-21-11-9-19-31(33)29-39-27-17-7-5-15-25-37-23-13-3-1-2-4-14-24-38-26-16-6-8-18-28-40-30-32-20-10-12-22-34(32)36/h9-12,19-22,37-40H,1-8,13-18,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405745

(CHEMBL5285879)Show SMILES COc1ccccc1CNCCCCCNCCCCCCCCNCCCCCNCc1ccccc1OC Show InChI InChI=1S/C34H58N4O2/c1-39-33-21-11-9-19-31(33)29-37-27-17-7-15-25-35-23-13-5-3-4-6-14-24-36-26-16-8-18-28-38-30-32-20-10-12-22-34(32)40-2/h9-12,19-22,35-38H,3-8,13-18,23-30H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405739

(CHEMBL5273396)Show SMILES Cc1ccccc1CNCCCCCCNCCCCCCCCNCCCCCCNCc1ccccc1C Show InChI InChI=1S/C36H62N4/c1-33-21-11-13-23-35(33)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-36-24-14-12-22-34(36)2/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405740
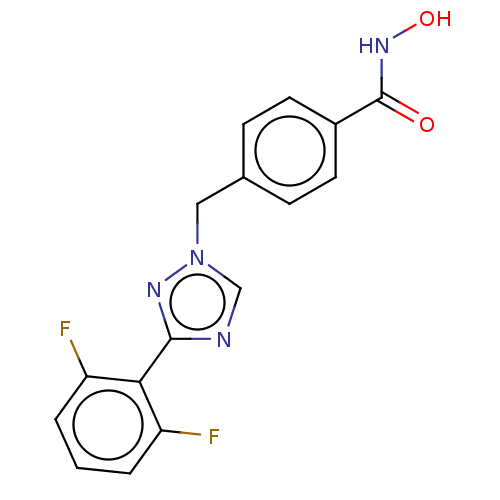
(CHEMBL5280669)Show SMILES COc1ccccc1CN(C)CCCCCCN(C)CCCCCCCCN(C)CCCCCCN(C)Cc1ccccc1OC Show InChI InChI=1S/C40H70N4O2/c1-41(31-21-11-13-23-33-43(3)35-37-25-15-17-27-39(37)45-5)29-19-9-7-8-10-20-30-42(2)32-22-12-14-24-34-44(4)36-38-26-16-18-28-40(38)46-6/h15-18,25-28H,7-14,19-24,29-36H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405743

(CHEMBL5274632)Show SMILES C(CCCCNCCCCCCNCc1ccccc1)CCCNCCCCCCNCc1ccccc1 Show InChI InChI=1S/C34H58N4/c1(3-15-25-35-27-17-5-7-19-29-37-31-33-21-11-9-12-22-33)2-4-16-26-36-28-18-6-8-20-30-38-32-34-23-13-10-14-24-34/h9-14,21-24,35-38H,1-8,15-20,25-32H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405756
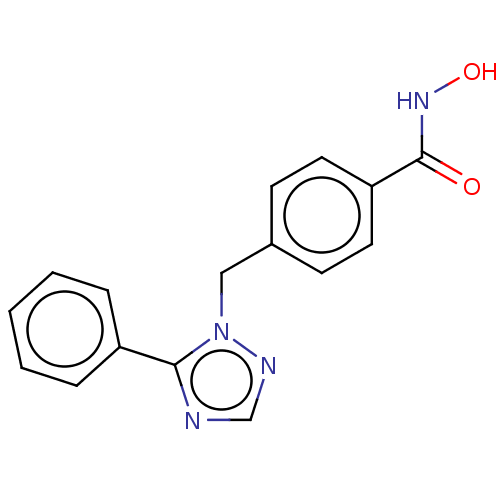
(CHEMBL5284689)Show SMILES C(CCCCNCCCCCCNCc1cccnc1)CCCNCCCCCCNCc1cccnc1 Show InChI InChI=1S/C32H56N6/c1(3-9-19-33-21-11-5-7-13-23-35-27-31-17-15-25-37-29-31)2-4-10-20-34-22-12-6-8-14-24-36-28-32-18-16-26-38-30-32/h15-18,25-26,29-30,33-36H,1-14,19-24,27-28H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405758
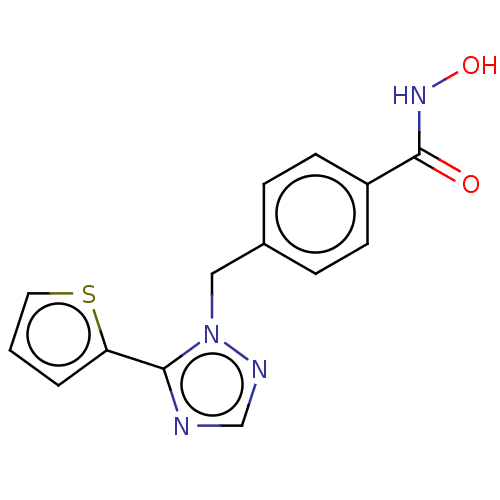
(CHEMBL5287109)Show SMILES Fc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H56F2N4/c35-33-19-15-31(16-20-33)29-39-27-13-7-5-11-25-37-23-9-3-1-2-4-10-24-38-26-12-6-8-14-28-40-30-32-17-21-34(36)22-18-32/h15-22,37-40H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405741
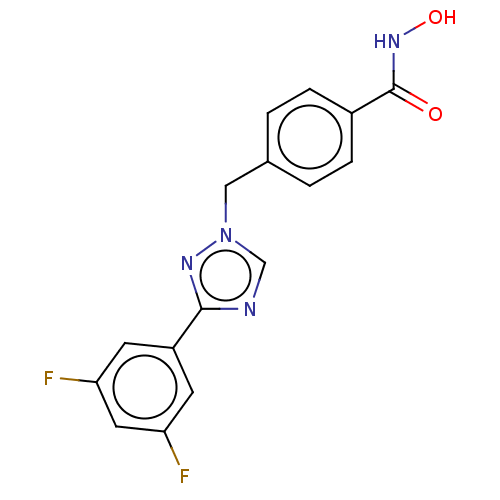
(CHEMBL5271970)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCCCCCCCNCCCCCCNCc1ccccc1C Show InChI InChI=1S/C42H74N4O/c1-39-27-17-18-28-40(39)37-45-35-25-15-13-23-33-43-31-21-11-9-7-5-3-4-6-8-10-12-22-32-44-34-24-14-16-26-36-46-38-41-29-19-20-30-42(41)47-2/h17-20,27-30,43-46H,3-16,21-26,31-38H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against thymidylate synthase isolated from L1210 leukemia cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405752
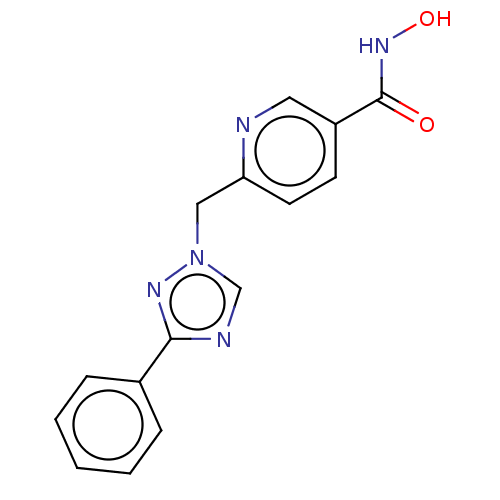
(CHEMBL5290601)Show SMILES C(CCCCNCCCCCCNCc1ccccn1)CCCNCCCCCCNCc1ccccn1 Show InChI InChI=1S/C32H56N6/c1(3-11-21-33-23-13-5-7-15-25-35-29-31-19-9-17-27-37-31)2-4-12-22-34-24-14-6-8-16-26-36-30-32-20-10-18-28-38-32/h9-10,17-20,27-28,33-36H,1-8,11-16,21-26,29-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405749
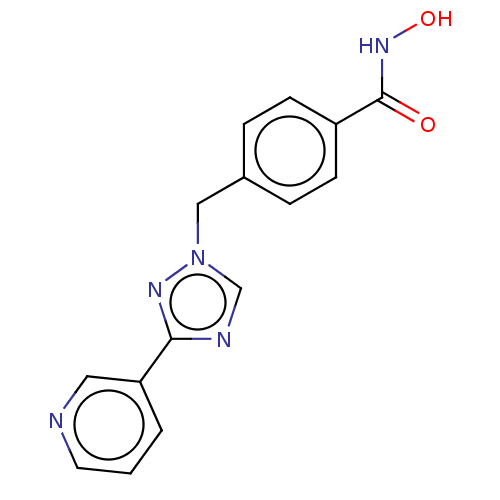
(CHEMBL5285996)Show SMILES Cc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(C)o2)o1 Show InChI InChI=1S/C32H58N4O2/c1-29-17-19-31(37-29)27-35-25-15-9-7-13-23-33-21-11-5-3-4-6-12-22-34-24-14-8-10-16-26-36-28-32-20-18-30(2)38-32/h17-20,33-36H,3-16,21-28H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405678
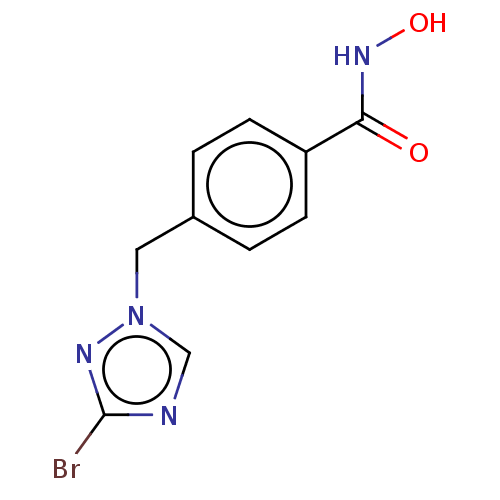
(CHEMBL5287254)Show InChI InChI=1S/C19H18NO/c1-20-14-6-5-7-16(20)12-10-15-11-13-19(21-2)18-9-4-3-8-17(15)18/h3-14H,1-2H3/q+1/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405677
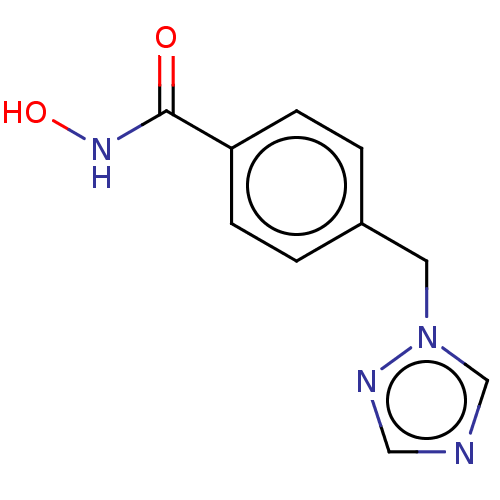
(CHEMBL5267379)Show SMILES C[N+]1(C)CCC(\C=C\c2cccc3ccccc23)=CC1 |c:19| Show InChI InChI=1S/C19H22N/c1-20(2)14-12-16(13-15-20)10-11-18-8-5-7-17-6-3-4-9-19(17)18/h3-12H,13-15H2,1-2H3/q+1/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) obtained from human WIL2 cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405742

(CHEMBL5271520)Show SMILES COc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(OC)cc2)cc1 Show InChI InChI=1S/C36H62N4O2/c1-41-35-21-17-33(18-22-35)31-39-29-15-9-7-13-27-37-25-11-5-3-4-6-12-26-38-28-14-8-10-16-30-40-32-34-19-23-36(42-2)24-20-34/h17-24,37-40H,3-16,25-32H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405744

(CHEMBL5274088)Show SMILES COc1ccccc1CNCCCCCCN(C)CCCCCCCCN(C)CCCCCCNCc1ccccc1OC Show InChI InChI=1S/C38H66N4O2/c1-41(31-21-11-7-17-27-39-33-35-23-13-15-25-37(35)43-3)29-19-9-5-6-10-20-30-42(2)32-22-12-8-18-28-40-34-36-24-14-16-26-38(36)44-4/h13-16,23-26,39-40H,5-12,17-22,27-34H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405748

(CHEMBL5279952)Show SMILES COc1ccccc1CNCCCCCCCNCCCCCCCCNCCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C38H66N4O2/c1-43-37-25-15-13-23-35(37)33-41-31-21-11-5-9-19-29-39-27-17-7-3-4-8-18-28-40-30-20-10-6-12-22-32-42-34-36-24-14-16-26-38(36)44-2/h13-16,23-26,39-42H,3-12,17-22,27-34H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50405755
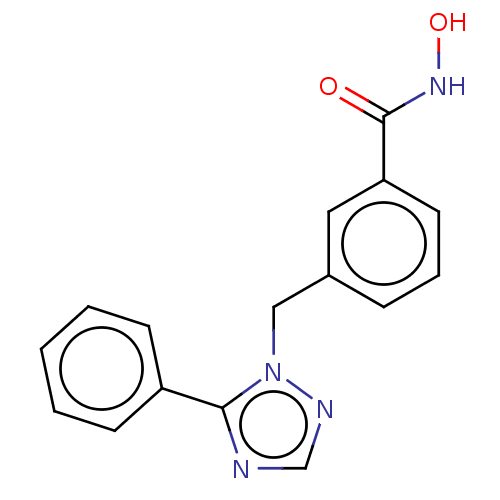
(CHEMBL5281671)Show SMILES COc1ccccc1CN(C)CCCCCCNCCCCCCCCNCCCCCCN(C)Cc1ccccc1OC Show InChI InChI=1S/C38H66N4O2/c1-41(33-35-23-13-15-25-37(35)43-3)31-21-11-9-19-29-39-27-17-7-5-6-8-18-28-40-30-20-10-12-22-32-42(2)34-36-24-14-16-26-38(36)44-4/h13-16,23-26,39-40H,5-12,17-22,27-34H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405746

(CHEMBL5286013)Show SMILES [O-][N+](=O)c1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C34H56N6O4/c41-39(42)33-19-15-31(16-20-33)29-37-27-13-7-5-11-25-35-23-9-3-1-2-4-10-24-36-26-12-6-8-14-28-38-30-32-17-21-34(22-18-32)40(43)44/h15-22,35-38H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405743

(CHEMBL5274632)Show SMILES C(CCCCNCCCCCCNCc1ccccc1)CCCNCCCCCCNCc1ccccc1 Show InChI InChI=1S/C34H58N4/c1(3-15-25-35-27-17-5-7-19-29-37-31-33-21-11-9-12-22-33)2-4-16-26-36-28-18-6-8-20-30-38-32-34-23-13-10-14-24-34/h9-14,21-24,35-38H,1-8,15-20,25-32H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405747

(CHEMBL5267739)Show SMILES Cc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(C)cc2)cc1 Show InChI InChI=1S/C36H62N4/c1-33-17-21-35(22-18-33)31-39-29-15-9-7-13-27-37-25-11-5-3-4-6-12-26-38-28-14-8-10-16-30-40-32-36-23-19-34(2)20-24-36/h17-24,37-40H,3-16,25-32H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405679

(CHEMBL5266236)Show InChI InChI=1S/C18H21N/c1-19-13-11-15(12-14-19)9-10-17-7-4-6-16-5-2-3-8-18(16)17/h2-10,15H,11-14H2,1H3/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405745

(CHEMBL5285879)Show SMILES COc1ccccc1CNCCCCCNCCCCCCCCNCCCCCNCc1ccccc1OC Show InChI InChI=1S/C34H58N4O2/c1-39-33-21-11-9-19-31(33)29-37-27-17-7-15-25-35-23-13-5-3-4-6-14-24-36-26-16-8-18-28-38-30-32-20-10-12-22-34(32)40-2/h9-12,19-22,35-38H,3-8,13-18,23-30H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405750

(CHEMBL5291256)Show InChI InChI=1S/C30H54N4O2/c1(3-9-19-31-21-11-5-7-13-23-33-27-29-17-15-25-35-29)2-4-10-20-32-22-12-6-8-14-24-34-28-30-18-16-26-36-30/h15-18,25-26,31-34H,1-14,19-24,27-28H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405680

(CHEMBL5268616)Show InChI InChI=1S/C18H16N/c1-19-14-5-4-10-17(19)13-12-16-9-6-8-15-7-2-3-11-18(15)16/h2-14H,1H3/q+1/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405741

(CHEMBL5271970)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCCCCCCCNCCCCCCNCc1ccccc1C Show InChI InChI=1S/C42H74N4O/c1-39-27-17-18-28-40(39)37-45-35-25-15-13-23-33-43-31-21-11-9-7-5-3-4-6-8-10-12-22-32-44-34-24-14-16-26-36-46-38-41-29-19-20-30-42(41)47-2/h17-20,27-30,43-46H,3-16,21-26,31-38H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405681

(CHEMBL5271793)Show InChI InChI=1S/C14H14N/c1-15-11-9-14(10-12-15)8-7-13-5-3-2-4-6-13/h2-12H,1H3/q+1/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405739

(CHEMBL5273396)Show SMILES Cc1ccccc1CNCCCCCCNCCCCCCCCNCCCCCCNCc1ccccc1C Show InChI InChI=1S/C36H62N4/c1-33-21-11-13-23-35(33)31-39-29-19-9-7-17-27-37-25-15-5-3-4-6-16-26-38-28-18-8-10-20-30-40-32-36-24-14-12-22-34(36)2/h11-14,21-24,37-40H,3-10,15-20,25-32H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against acetylcholinetransferase (AChE) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405753

(CHEMBL5274634)Show SMILES Clc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C34H56Cl2N4/c35-33-19-15-31(16-20-33)29-39-27-13-7-5-11-25-37-23-9-3-1-2-4-10-24-38-26-12-6-8-14-28-40-30-32-17-21-34(36)22-18-32/h15-22,37-40H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405753

(CHEMBL5274634)Show SMILES Clc1ccc(CNCCCCCCNCCCCCCCCNCCCCCCNCc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C34H56Cl2N4/c35-33-19-15-31(16-20-33)29-39-27-13-7-5-11-25-37-23-9-3-1-2-4-10-24-38-26-12-6-8-14-28-40-30-32-17-21-34(36)22-18-32/h15-22,37-40H,1-14,23-30H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405756

(CHEMBL5284689)Show SMILES C(CCCCNCCCCCCNCc1cccnc1)CCCNCCCCCCNCc1cccnc1 Show InChI InChI=1S/C32H56N6/c1(3-9-19-33-21-11-5-7-13-23-35-27-31-17-15-25-37-29-31)2-4-10-20-34-22-12-6-8-14-24-36-28-32-18-16-26-38-30-32/h15-18,25-26,29-30,33-36H,1-14,19-24,27-28H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (CAT) enzyme |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data