 Found 1337 hits of ic50 data for polymerid = 1932,1933,1934,1935,1936,50000048,50000343,50004802,50004818,50004819,50005738
Found 1337 hits of ic50 data for polymerid = 1932,1933,1934,1935,1936,50000048,50000343,50004802,50004818,50004819,50005738 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Lactobacillus casei) | BDBM50226275
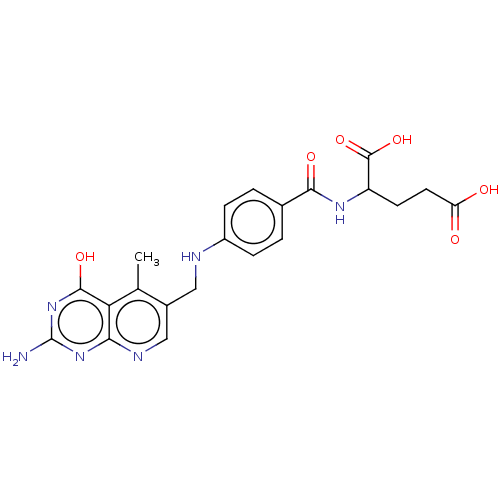
(CHEMBL267104)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(O)c12 Show InChI InChI=1S/C21H22N6O6/c1-10-12(9-24-17-16(10)19(31)27-21(22)26-17)8-23-13-4-2-11(3-5-13)18(30)25-14(20(32)33)6-7-15(28)29/h2-5,9,14,23H,6-8H2,1H3,(H,25,30)(H,28,29)(H,32,33)(H3,22,24,26,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 ... |
Eur J Med Chem 46: 993-1005 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.005
BindingDB Entry DOI: 10.7270/Q2X34XS1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50288983

((S)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H32FN5O9/c1-4-11-37(15-18-13-21-25(12-16(18)2)33-17(3)34-29(21)42)19-5-6-20(22(32)14-19)28(41)36-24(31(45)46)7-9-26(38)35-23(30(43)44)8-10-27(39)40/h1,5-6,12-14,23-24H,7-11,15H2,2-3H3,(H,35,38)(H,36,41)(H,39,40)(H,43,44)(H,45,46)(H,33,34,42)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Thymidylate synthase in experiment 1 |
Bioorg Med Chem Lett 6: 631-636 (1996)
Article DOI: 10.1016/0960-894X(96)00078-9
BindingDB Entry DOI: 10.7270/Q2XS5VC3 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049159
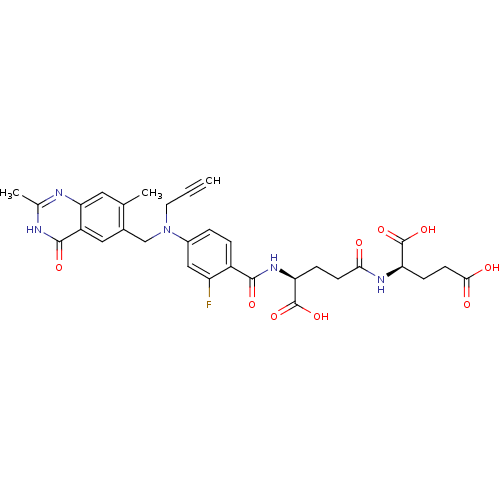
((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H32FN5O9/c1-4-11-37(15-18-13-21-25(12-16(18)2)33-17(3)34-29(21)42)19-5-6-20(22(32)14-19)28(41)36-24(31(45)46)7-9-26(38)35-23(30(43)44)8-10-27(39)40/h1,5-6,12-14,23-24H,7-11,15H2,2-3H3,(H,35,38)(H,36,41)(H,39,40)(H,43,44)(H,45,46)(H,33,34,42)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 h... |
Eur J Med Chem 46: 993-1005 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.005
BindingDB Entry DOI: 10.7270/Q2X34XS1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50288983

((S)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H32FN5O9/c1-4-11-37(15-18-13-21-25(12-16(18)2)33-17(3)34-29(21)42)19-5-6-20(22(32)14-19)28(41)36-24(31(45)46)7-9-26(38)35-23(30(43)44)8-10-27(39)40/h1,5-6,12-14,23-24H,7-11,15H2,2-3H3,(H,35,38)(H,36,41)(H,39,40)(H,43,44)(H,45,46)(H,33,34,42)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Thymidylate synthase in experiment 1 |
Bioorg Med Chem Lett 6: 631-636 (1996)
Article DOI: 10.1016/0960-894X(96)00078-9
BindingDB Entry DOI: 10.7270/Q2XS5VC3 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081273
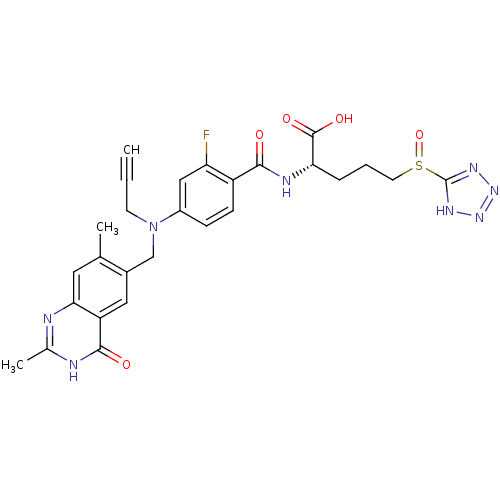
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCS(=O)c4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C27H27FN8O5S/c1-4-9-36(14-17-12-20-23(11-15(17)2)29-16(3)30-25(20)38)18-7-8-19(21(28)13-18)24(37)31-22(26(39)40)6-5-10-42(41)27-32-34-35-33-27/h1,7-8,11-13,22H,5-6,9-10,14H2,2-3H3,(H,31,37)(H,39,40)(H,29,30,38)(H,32,33,34,35)/t22-,42?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049168
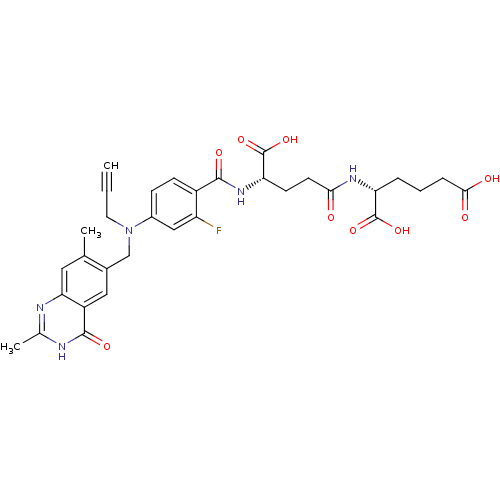
((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@H](CCCC(O)=O)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C32H34FN5O9/c1-4-12-38(16-19-14-22-26(13-17(19)2)34-18(3)35-30(22)43)20-8-9-21(23(33)15-20)29(42)37-25(32(46)47)10-11-27(39)36-24(31(44)45)6-5-7-28(40)41/h1,8-9,13-15,24-25H,5-7,10-12,16H2,2-3H3,(H,36,39)(H,37,42)(H,40,41)(H,44,45)(H,46,47)(H,34,35,43)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081256

((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCSc4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C27H27FN8O4S/c1-4-9-36(14-17-12-20-23(11-15(17)2)29-16(3)30-25(20)38)18-7-8-19(21(28)13-18)24(37)31-22(26(39)40)6-5-10-41-27-32-34-35-33-27/h1,7-8,11-13,22H,5-6,9-10,14H2,2-3H3,(H,31,37)(H,39,40)(H,29,30,38)(H,32,33,34,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 15 ... |
Eur J Med Chem 46: 993-1005 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.005
BindingDB Entry DOI: 10.7270/Q2X34XS1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 15... |
Eur J Med Chem 46: 993-1005 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.005
BindingDB Entry DOI: 10.7270/Q2X34XS1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081259

((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCS(=O)(=O)c4n[nH]c(=O)[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H28FN7O7S/c1-4-9-36(14-17-12-20-23(11-15(17)2)30-16(3)31-25(20)38)18-7-8-19(21(29)13-18)24(37)32-22(26(39)40)6-5-10-44(42,43)28-33-27(41)34-35-28/h1,7-8,11-13,22H,5-6,9-10,14H2,2-3H3,(H,32,37)(H,39,40)(H,30,31,38)(H2,33,34,35,41)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 ... |
Eur J Med Chem 46: 993-1005 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.005
BindingDB Entry DOI: 10.7270/Q2X34XS1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50340678

(1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cc(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24... |
Eur J Med Chem 46: 993-1005 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.005
BindingDB Entry DOI: 10.7270/Q2X34XS1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50288984
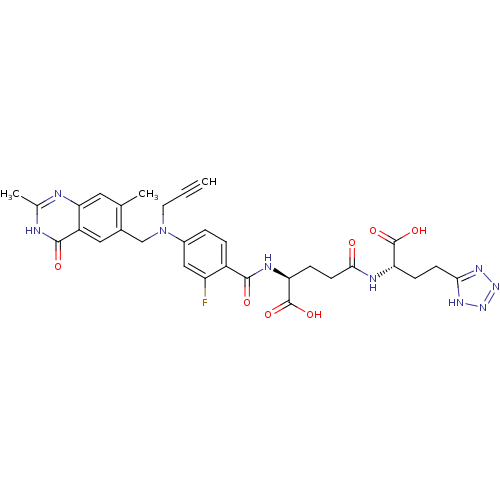
((S)-4-[(S)-1-Carboxy-3-(1H-tetrazol-5-yl)-propylca...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@@H](CCc4nnn[nH]4)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H32FN9O7/c1-4-11-41(15-18-13-21-25(12-16(18)2)33-17(3)34-29(21)44)19-5-6-20(22(32)14-19)28(43)36-24(31(47)48)8-10-27(42)35-23(30(45)46)7-9-26-37-39-40-38-26/h1,5-6,12-14,23-24H,7-11,15H2,2-3H3,(H,35,42)(H,36,43)(H,45,46)(H,47,48)(H,33,34,44)(H,37,38,39,40)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Thymidylate synthase in experiment 1 |
Bioorg Med Chem Lett 6: 631-636 (1996)
Article DOI: 10.1016/0960-894X(96)00078-9
BindingDB Entry DOI: 10.7270/Q2XS5VC3 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50288984

((S)-4-[(S)-1-Carboxy-3-(1H-tetrazol-5-yl)-propylca...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@@H](CCc4nnn[nH]4)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H32FN9O7/c1-4-11-41(15-18-13-21-25(12-16(18)2)33-17(3)34-29(21)44)19-5-6-20(22(32)14-19)28(43)36-24(31(47)48)8-10-27(42)35-23(30(45)46)7-9-26-37-39-40-38-26/h1,5-6,12-14,23-24H,7-11,15H2,2-3H3,(H,35,42)(H,36,43)(H,45,46)(H,47,48)(H,33,34,44)(H,37,38,39,40)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Thymidylate synthase in experiment 1 |
Bioorg Med Chem Lett 6: 631-636 (1996)
Article DOI: 10.1016/0960-894X(96)00078-9
BindingDB Entry DOI: 10.7270/Q2XS5VC3 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081265
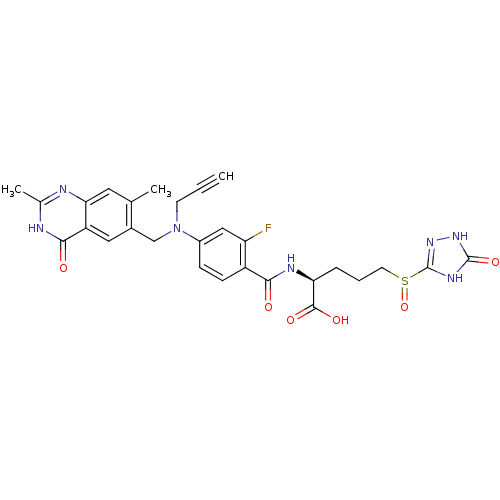
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCS(=O)c4n[nH]c(=O)[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H28FN7O6S/c1-4-9-36(14-17-12-20-23(11-15(17)2)30-16(3)31-25(20)38)18-7-8-19(21(29)13-18)24(37)32-22(26(39)40)6-5-10-43(42)28-33-27(41)34-35-28/h1,7-8,11-13,22H,5-6,9-10,14H2,2-3H3,(H,32,37)(H,39,40)(H,30,31,38)(H2,33,34,35,41)/t22-,43?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50288985
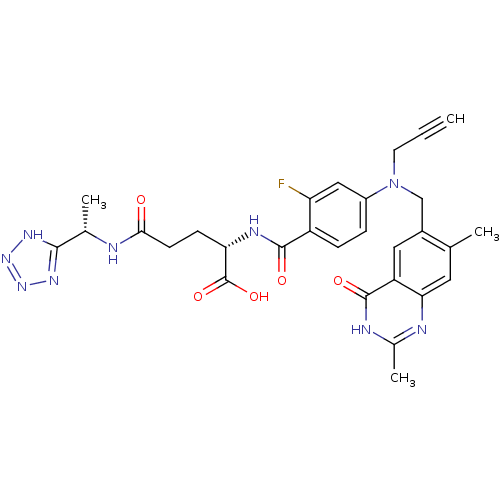
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES C[C@H](NC(=O)CC[C@H](NC(=O)c1ccc(cc1F)N(CC#C)Cc1cc2c(cc1C)nc(C)[nH]c2=O)C(O)=O)c1nnn[nH]1 Show InChI InChI=1S/C29H30FN9O5/c1-5-10-39(14-18-12-21-24(11-15(18)2)32-17(4)33-28(21)42)19-6-7-20(22(30)13-19)27(41)34-23(29(43)44)8-9-25(40)31-16(3)26-35-37-38-36-26/h1,6-7,11-13,16,23H,8-10,14H2,2-4H3,(H,31,40)(H,34,41)(H,43,44)(H,32,33,42)(H,35,36,37,38)/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Thymidylate synthase. |
Bioorg Med Chem Lett 6: 631-636 (1996)
Article DOI: 10.1016/0960-894X(96)00078-9
BindingDB Entry DOI: 10.7270/Q2XS5VC3 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50326712
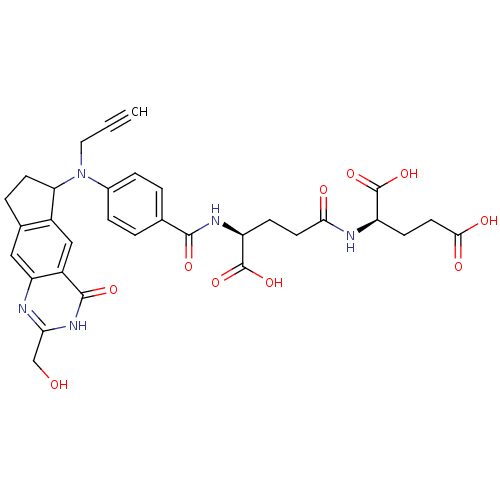
(2(R)-[4(S)-carboxy-4-[4-[N-(2-hydroxymethyl-4-oxo-...)Show SMILES OCc1nc2cc3CCC(N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c3cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C32H33N5O10/c1-2-13-37(25-10-5-18-14-24-21(15-20(18)25)30(43)36-26(16-38)33-24)19-6-3-17(4-7-19)29(42)35-23(32(46)47)8-11-27(39)34-22(31(44)45)9-12-28(40)41/h1,3-4,6-7,14-15,22-23,25,38H,5,8-13,16H2,(H,34,39)(H,35,42)(H,40,41)(H,44,45)(H,46,47)(H,33,36,43)/t22-,23+,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH
Curated by ChEMBL
| Assay Description
Inhibition of thymidin synthase |
J Med Chem 53: 6539-49 (2010)
Article DOI: 10.1021/jm901869w
BindingDB Entry DOI: 10.7270/Q24J0F9S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Mus musculus) | BDBM50081240
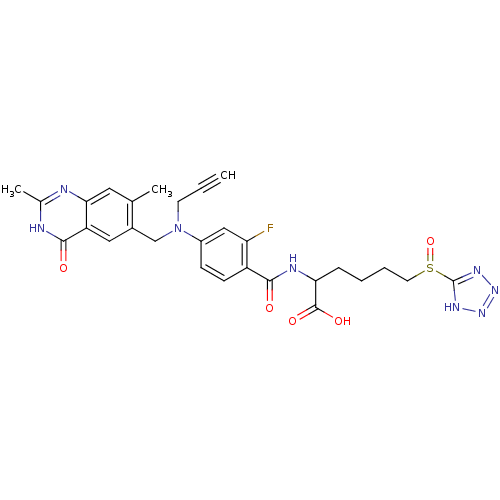
(2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)NC(CCCCS(=O)c4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H29FN8O5S/c1-4-10-37(15-18-13-21-24(12-16(18)2)30-17(3)31-26(21)39)19-8-9-20(22(29)14-19)25(38)32-23(27(40)41)7-5-6-11-43(42)28-33-35-36-34-28/h1,8-9,12-14,23H,5-7,10-11,15H2,2-3H3,(H,32,38)(H,40,41)(H,30,31,39)(H,33,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081246
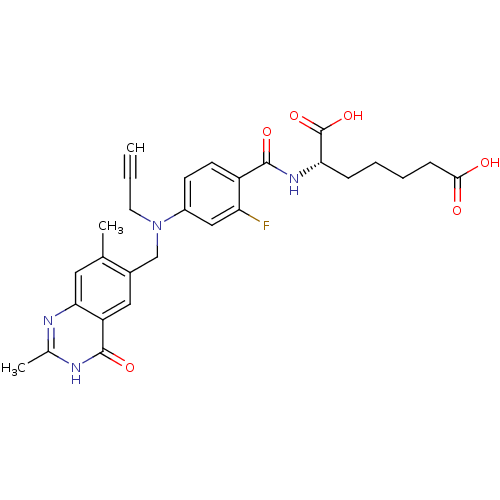
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H29FN4O6/c1-4-11-33(15-18-13-21-24(12-16(18)2)30-17(3)31-27(21)37)19-9-10-20(22(29)14-19)26(36)32-23(28(38)39)7-5-6-8-25(34)35/h1,9-10,12-14,23H,5-8,11,15H2,2-3H3,(H,32,36)(H,34,35)(H,38,39)(H,30,31,37)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081252
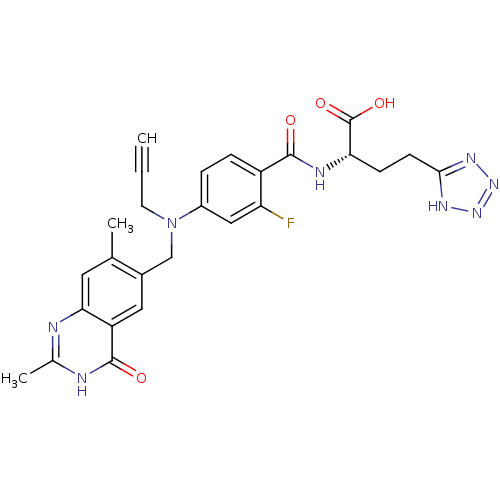
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCc4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H25FN8O4/c1-4-9-35(13-16-11-19-22(10-14(16)2)28-15(3)29-25(19)37)17-5-6-18(20(27)12-17)24(36)30-21(26(38)39)7-8-23-31-33-34-32-23/h1,5-6,10-12,21H,7-9,13H2,2-3H3,(H,30,36)(H,38,39)(H,28,29,37)(H,31,32,33,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081243
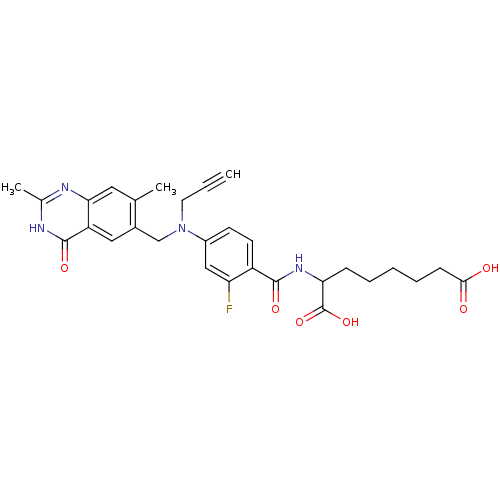
(2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)NC(CCCCCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C29H31FN4O6/c1-4-12-34(16-19-14-22-25(13-17(19)2)31-18(3)32-28(22)38)20-10-11-21(23(30)15-20)27(37)33-24(29(39)40)8-6-5-7-9-26(35)36/h1,10-11,13-15,24H,5-9,12,16H2,2-3H3,(H,33,37)(H,35,36)(H,39,40)(H,31,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081260
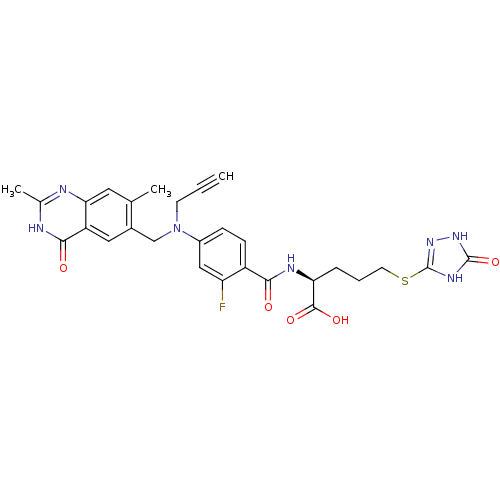
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCSc4n[nH]c(=O)[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H28FN7O5S/c1-4-9-36(14-17-12-20-23(11-15(17)2)30-16(3)31-25(20)38)18-7-8-19(21(29)13-18)24(37)32-22(26(39)40)6-5-10-42-28-33-27(41)34-35-28/h1,7-8,11-13,22H,5-6,9-10,14H2,2-3H3,(H,32,37)(H,39,40)(H,30,31,38)(H2,33,34,35,41)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081261
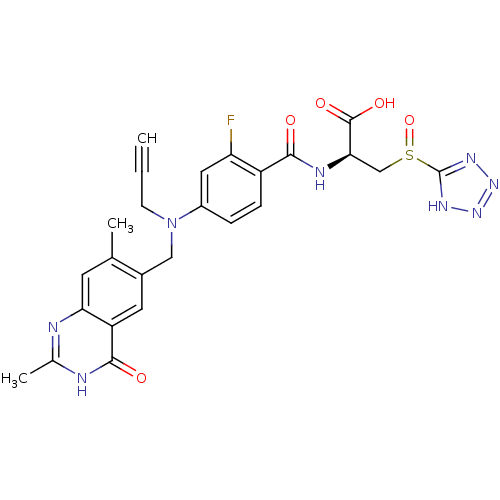
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@H](CS(=O)c4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H23FN8O5S/c1-4-7-34(11-15-9-18-20(8-13(15)2)27-14(3)28-23(18)36)16-5-6-17(19(26)10-16)22(35)29-21(24(37)38)12-40(39)25-30-32-33-31-25/h1,5-6,8-10,21H,7,11-12H2,2-3H3,(H,29,35)(H,37,38)(H,27,28,36)(H,30,31,32,33)/t21-,40?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50107343
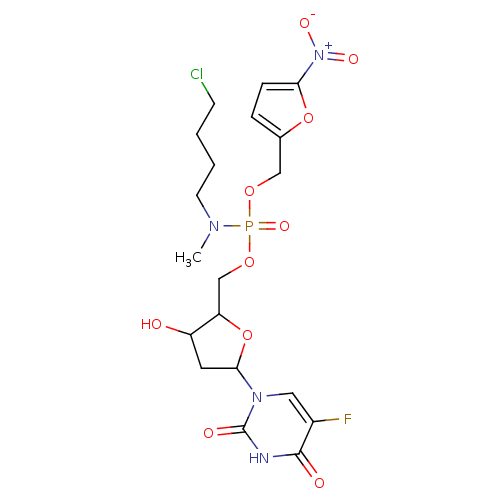
((4-Chloro-butyl)-methyl-phosphoramidic acid 5-(5-f...)Show SMILES CN(CCCCCl)P(=O)(OCC1OC(CC1O)n1cc(F)c(=O)[nH]c1=O)OCc1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C19H25ClFN4O10P/c1-23(7-3-2-6-20)36(31,32-10-12-4-5-16(34-12)25(29)30)33-11-15-14(26)8-17(35-15)24-9-13(21)18(27)22-19(24)28/h4-5,9,14-15,17,26H,2-3,6-8,10-11H2,1H3,(H,22,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Thymidylate synthase inhibition in thymidine kinase deficient /TK cells after 2 h treatment |
J Med Chem 44: 4475-80 (2001)
BindingDB Entry DOI: 10.7270/Q2HM57R4 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081247
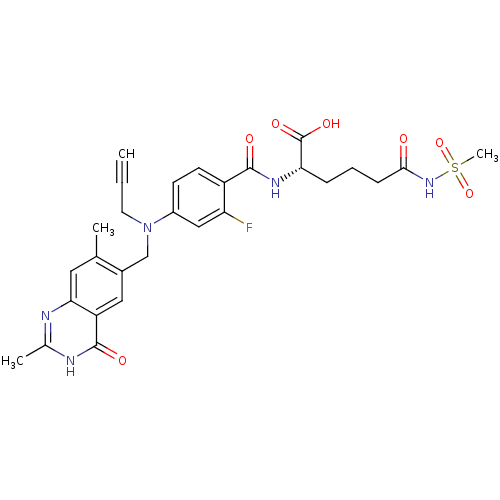
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCC(=O)NS(C)(=O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H30FN5O7S/c1-5-11-34(15-18-13-21-24(12-16(18)2)30-17(3)31-27(21)37)19-9-10-20(22(29)14-19)26(36)32-23(28(38)39)7-6-8-25(35)33-42(4,40)41/h1,9-10,12-14,23H,6-8,11,15H2,2-4H3,(H,32,36)(H,33,35)(H,38,39)(H,30,31,37)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049170

((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H33N5O9/c1-4-13-36(16-20-15-22-25(14-17(20)2)32-18(3)33-29(22)41)21-7-5-19(6-8-21)28(40)35-24(31(44)45)9-11-26(37)34-23(30(42)43)10-12-27(38)39/h1,5-8,14-15,23-24H,9-13,16H2,2-3H3,(H,34,37)(H,35,40)(H,38,39)(H,42,43)(H,44,45)(H,32,33,41)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049172
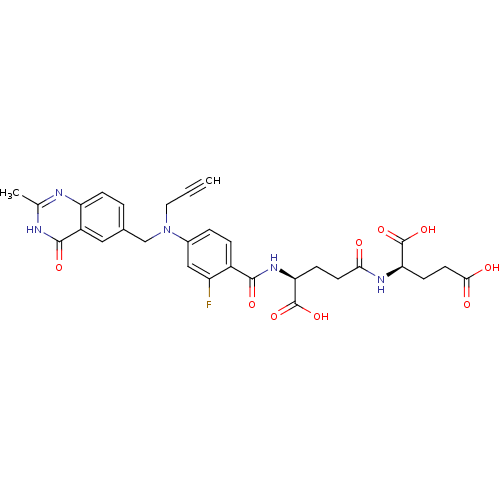
((R)-2-((S)-4-Carboxy-4-{2-fluoro-4-[(2-methyl-4-ox...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C30H30FN5O9/c1-3-12-36(15-17-4-7-22-20(13-17)28(41)33-16(2)32-22)18-5-6-19(21(31)14-18)27(40)35-24(30(44)45)8-10-25(37)34-23(29(42)43)9-11-26(38)39/h1,4-7,13-14,23-24H,8-12,15H2,2H3,(H,34,37)(H,35,40)(H,38,39)(H,42,43)(H,44,45)(H,32,33,41)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50037316

((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@H](CCC(=O)N[C@H](CCCC(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H33N5O9/c1-3-15-36(17-19-7-12-23-22(16-19)29(41)33-18(2)32-23)21-10-8-20(9-11-21)28(40)35-25(31(44)45)13-14-26(37)34-24(30(42)43)5-4-6-27(38)39/h1,7-12,16,24-25H,4-6,13-15,17H2,2H3,(H,34,37)(H,35,40)(H,38,39)(H,42,43)(H,44,45)(H,32,33,41)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells |
J Med Chem 37: 3294-302 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3N50 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50037303

((R)-2-((R)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C30H31N5O9/c1-3-14-35(16-18-4-9-22-21(15-18)28(40)32-17(2)31-22)20-7-5-19(6-8-20)27(39)34-24(30(43)44)10-12-25(36)33-23(29(41)42)11-13-26(37)38/h1,4-9,15,23-24H,10-14,16H2,2H3,(H,33,36)(H,34,39)(H,37,38)(H,41,42)(H,43,44)(H,31,32,40)/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Tested for inhibition against thymidylate synthase(TS) which is partially purified from L1210 mouse leukemia cells |
J Med Chem 37: 3294-302 (1994)
BindingDB Entry DOI: 10.7270/Q2QV3N50 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049175
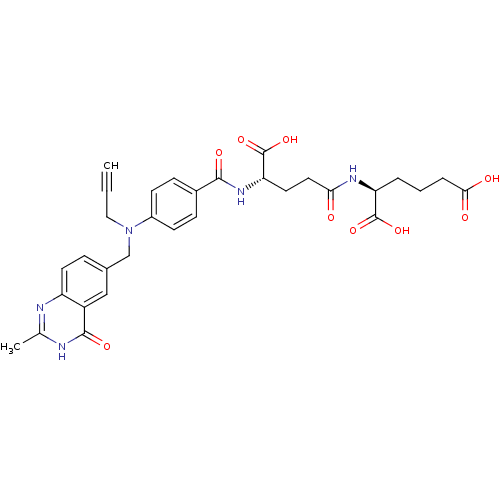
((S)-2-((S)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(=O)N[C@@H](CCCC(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C31H33N5O9/c1-3-15-36(17-19-7-12-23-22(16-19)29(41)33-18(2)32-23)21-10-8-20(9-11-21)28(40)35-25(31(44)45)13-14-26(37)34-24(30(42)43)5-4-6-27(38)39/h1,7-12,16,24-25H,4-6,13-15,17H2,2H3,(H,34,37)(H,35,40)(H,38,39)(H,42,43)(H,44,45)(H,32,33,41)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049164
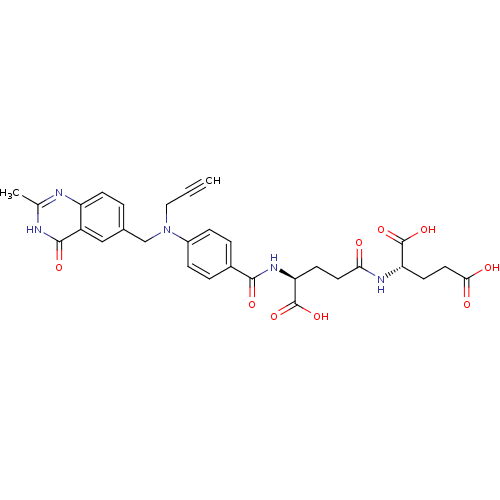
((S)-2-((S)-4-Carboxy-4-{4-[(2-methyl-4-oxo-3,4-dih...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C30H31N5O9/c1-3-14-35(16-18-4-9-22-21(15-18)28(40)32-17(2)31-22)20-7-5-19(6-8-20)27(39)34-24(30(43)44)10-12-25(36)33-23(29(41)42)11-13-26(37)38/h1,4-9,15,23-24H,10-14,16H2,2H3,(H,33,36)(H,34,39)(H,37,38)(H,41,42)(H,43,44)(H,31,32,40)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081239
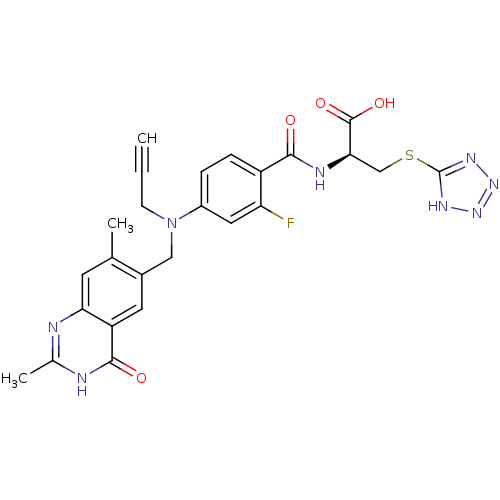
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@H](CSc4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H23FN8O4S/c1-4-7-34(11-15-9-18-20(8-13(15)2)27-14(3)28-23(18)36)16-5-6-17(19(26)10-16)22(35)29-21(24(37)38)12-39-25-30-32-33-31-25/h1,5-6,8-10,21H,7,11-12H2,2-3H3,(H,29,35)(H,37,38)(H,27,28,36)(H,30,31,32,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50049176
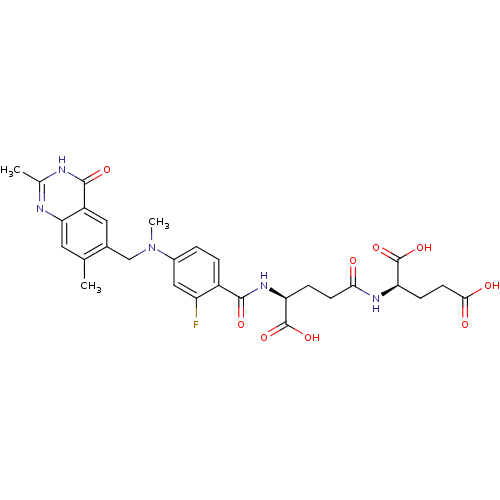
((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...)Show SMILES CN(Cc1cc2c(cc1C)nc(C)[nH]c2=O)c1ccc(C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c(F)c1 Show InChI InChI=1S/C29H32FN5O9/c1-14-10-23-19(27(40)32-15(2)31-23)11-16(14)13-35(3)17-4-5-18(20(30)12-17)26(39)34-22(29(43)44)6-8-24(36)33-21(28(41)42)7-9-25(37)38/h4-5,10-12,21-22H,6-9,13H2,1-3H3,(H,33,36)(H,34,39)(H,37,38)(H,41,42)(H,43,44)(H,31,32,40)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Thymidylate synthase activity in L1210 cells |
J Med Chem 39: 73-85 (1996)
Article DOI: 10.1021/jm950471+
BindingDB Entry DOI: 10.7270/Q2WH2P2S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081253
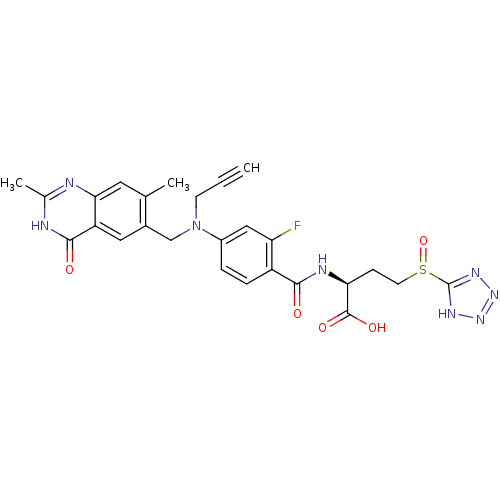
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCS(=O)c4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H25FN8O5S/c1-4-8-35(13-16-11-19-22(10-14(16)2)28-15(3)29-24(19)37)17-5-6-18(20(27)12-17)23(36)30-21(25(38)39)7-9-41(40)26-31-33-34-32-26/h1,5-6,10-12,21H,7-9,13H2,2-3H3,(H,30,36)(H,38,39)(H,28,29,37)(H,31,32,33,34)/t21-,41?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081272
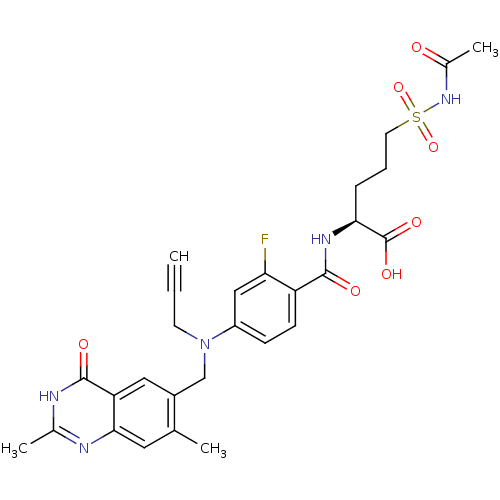
((S)-5-Acetylsulfamoyl-2-{4-[(2,7-dimethyl-4-oxo-3,...)Show SMILES CC(=O)NS(=O)(=O)CCC[C@H](NC(=O)c1ccc(cc1F)N(CC#C)Cc1cc2c(cc1C)nc(C)[nH]c2=O)C(O)=O Show InChI InChI=1S/C28H30FN5O7S/c1-5-10-34(15-19-13-22-25(12-16(19)2)30-17(3)31-27(22)37)20-8-9-21(23(29)14-20)26(36)32-24(28(38)39)7-6-11-42(40,41)33-18(4)35/h1,8-9,12-14,24H,6-7,10-11,15H2,2-4H3,(H,32,36)(H,33,35)(H,38,39)(H,30,31,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081249
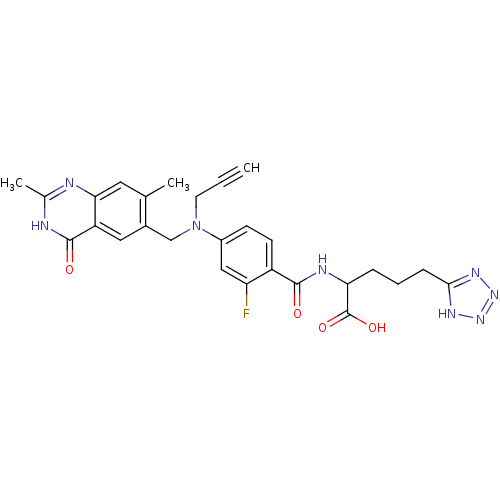
(2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin-6...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)NC(CCCc4nnn[nH]4)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C27H27FN8O4/c1-4-10-36(14-17-12-20-23(11-15(17)2)29-16(3)30-26(20)38)18-8-9-19(21(28)13-18)25(37)31-22(27(39)40)6-5-7-24-32-34-35-33-24/h1,8-9,11-13,22H,5-7,10,14H2,2-3H3,(H,31,37)(H,39,40)(H,29,30,38)(H,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50081264
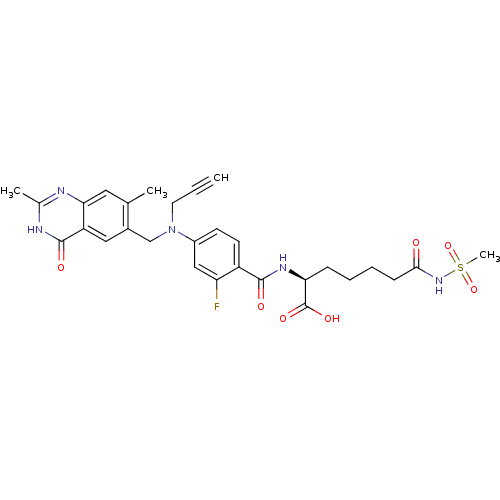
((S)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](CCCCC(=O)NS(C)(=O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C29H32FN5O7S/c1-5-12-35(16-19-14-22-25(13-17(19)2)31-18(3)32-28(22)38)20-10-11-21(23(30)15-20)27(37)33-24(29(39)40)8-6-7-9-26(36)34-43(4,41)42/h1,10-11,13-15,24H,6-9,12,16H2,2-4H3,(H,33,37)(H,34,36)(H,39,40)(H,31,32,38)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of isolated thymidylate synthase partially purified from L1210 mouse leukemia cells |
J Med Chem 42: 3809-20 (1999)
BindingDB Entry DOI: 10.7270/Q25M64XD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50226277
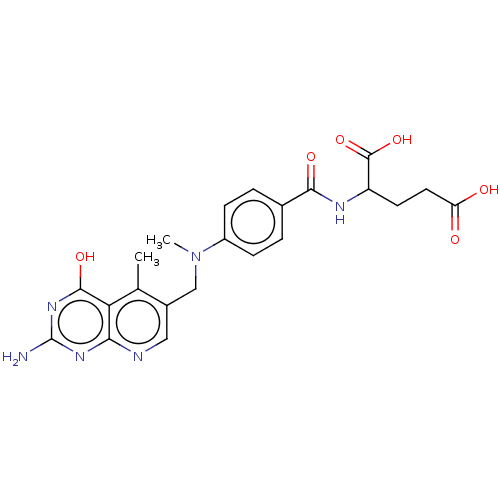
(CHEMBL273509)Show SMILES CN(Cc1cnc2nc(N)nc(O)c2c1C)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H24N6O6/c1-11-13(9-24-18-17(11)20(32)27-22(23)26-18)10-28(2)14-5-3-12(4-6-14)19(31)25-15(21(33)34)7-8-16(29)30/h3-6,9,15H,7-8,10H2,1-2H3,(H,25,31)(H,29,30)(H,33,34)(H3,23,24,26,27,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615588
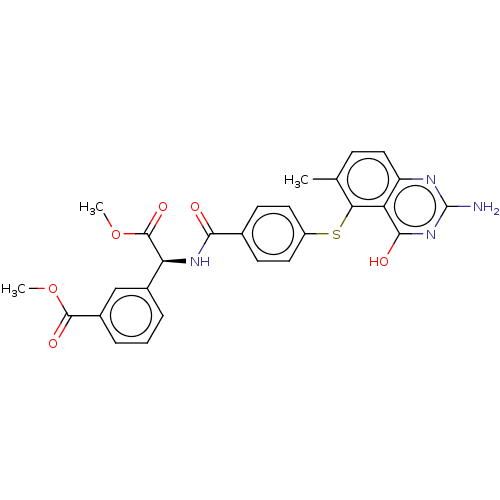
(CHEMBL320036)Show SMILES COC(=O)[C@@H](NC(=O)c1ccc(Sc2c(C)ccc3nc(N)nc(O)c23)cc1)c1cccc(c1)C(=O)OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615589
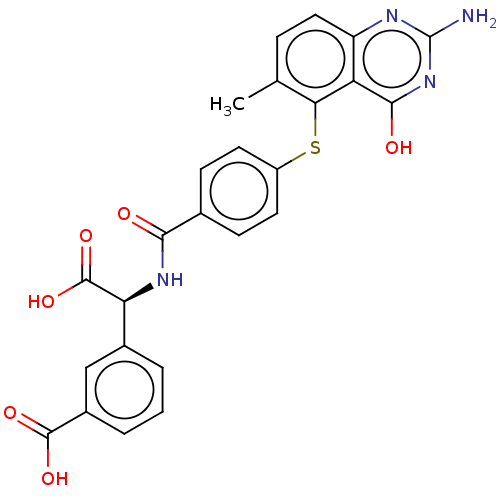
(CHEMBL264198)Show SMILES Cc1ccc2nc(N)nc(O)c2c1Sc1ccc(cc1)C(=O)N[C@H](C(O)=O)c1cccc(c1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615587

(CHEMBL109881)Show SMILES Cc1ccc2nc(N)nc(O)c2c1Sc1ccc(cc1)C(=O)N[C@H](C(O)=O)c1cccc(O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615586
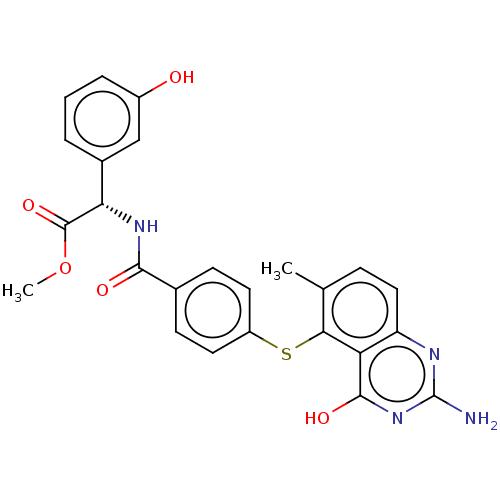
(CHEMBL113019)Show SMILES COC(=O)[C@@H](NC(=O)c1ccc(Sc2c(C)ccc3nc(N)nc(O)c23)cc1)c1cccc(O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615585

(CHEMBL111859)Show SMILES Cc1ccc2nc(N)nc(O)c2c1Sc1ccc(cc1)C(=O)N[C@H](C(O)=O)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615590
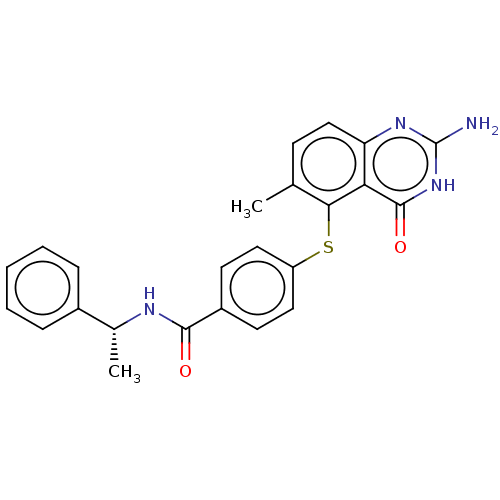
(CHEMBL5289121)Show SMILES C[C@@H](NC(=O)c1ccc(Sc2c(C)ccc3nc(N)[nH]c(=O)c23)cc1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615591
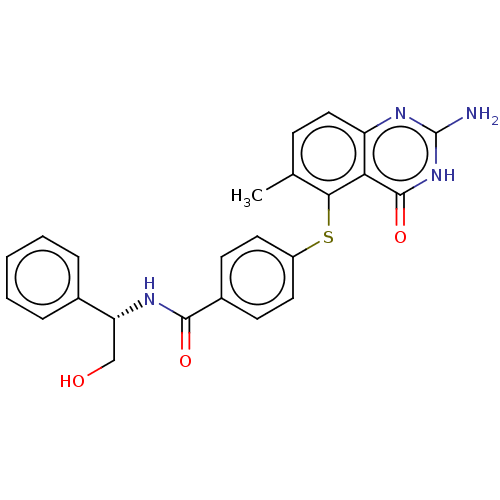
(CHEMBL5289689)Show SMILES Cc1ccc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@H](CO)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615592

(CHEMBL5283610)Show SMILES COC(=O)[C@@H](NC(=O)c1ccc(Sc2c(C)ccc3nc(N)[nH]c(=O)c23)s1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615593
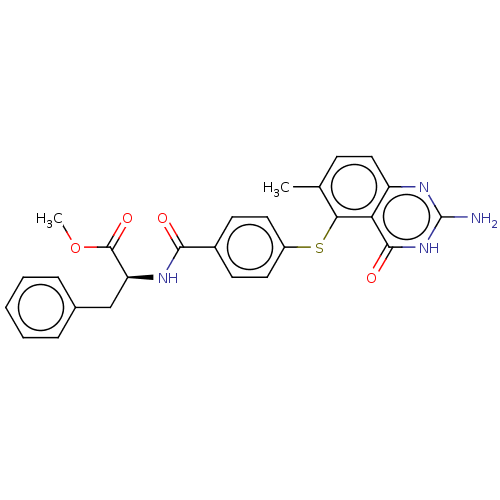
(CHEMBL5287291)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(Sc2c(C)ccc3nc(N)[nH]c(=O)c23)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data