 Found 220 hits of ic50 data for polymerid = 2043
Found 220 hits of ic50 data for polymerid = 2043 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764
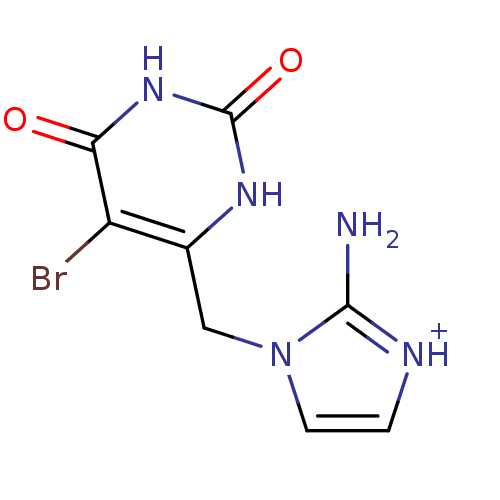
(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50274904
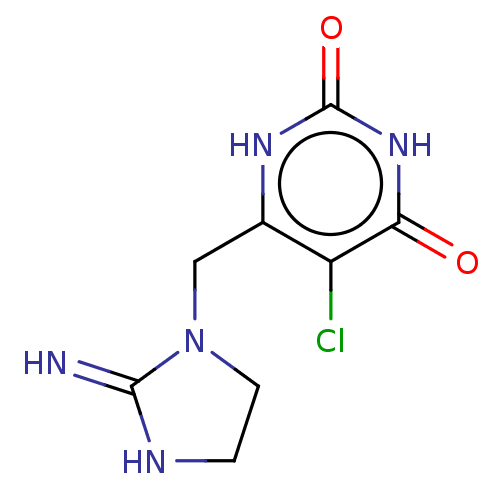
(CHEMBL4129617)Show InChI InChI=1S/C8H10ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-3H2,(H2,10,11)(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) |
Bioorg Med Chem 26: 3654-3663 (2018)
Article DOI: 10.1016/j.bmc.2018.05.046
BindingDB Entry DOI: 10.7270/Q22V2JKV |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079
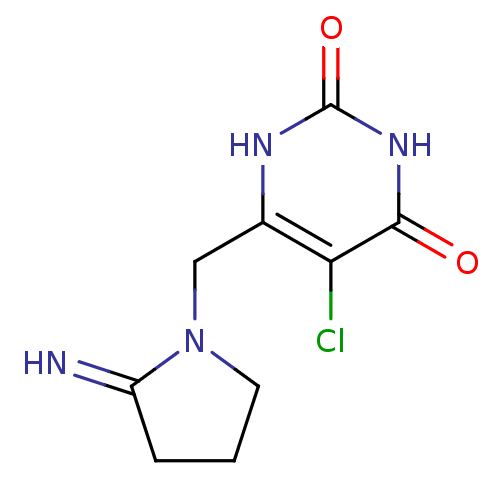
(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122771
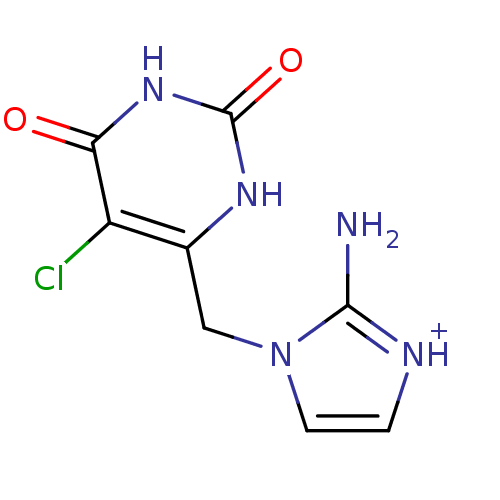
(2-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) using thymidine as substrate after 1 hr by spectrophotometric analysis |
Eur J Med Chem 144: 41-51 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.016
BindingDB Entry DOI: 10.7270/Q24M9762 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University
Curated by ChEMBL
| Assay Description
Inhibition of human placenta thymidine phosphorylase using [6-3H]dThd as substrate after 5 mins by scintillation counting method |
Bioorg Med Chem 26: 3654-3663 (2018)
Article DOI: 10.1016/j.bmc.2018.05.046
BindingDB Entry DOI: 10.7270/Q22V2JKV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AIMST University
Curated by ChEMBL
| Assay Description
Inhibition of human placenta thymidine phosphorylase using [6-3H]dThd as substrate after 5 mins by scintillation counting method |
Eur J Med Chem 124: 992-1003 (2016)
Article DOI: 10.1016/j.ejmech.2016.10.032
BindingDB Entry DOI: 10.7270/Q2RR218S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase TP |
Bioorg Med Chem Lett 13: 107-10 (2002)
BindingDB Entry DOI: 10.7270/Q2GM87V9 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase |
Eur J Med Chem 65: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.063
BindingDB Entry DOI: 10.7270/Q2125V2R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Competitive inhibition of human thymidine phosphorylase in presence of thymidine |
Eur J Med Chem 70: 400-10 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.022
BindingDB Entry DOI: 10.7270/Q2CZ38NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase by [3H]thymidine incorporation assay |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770
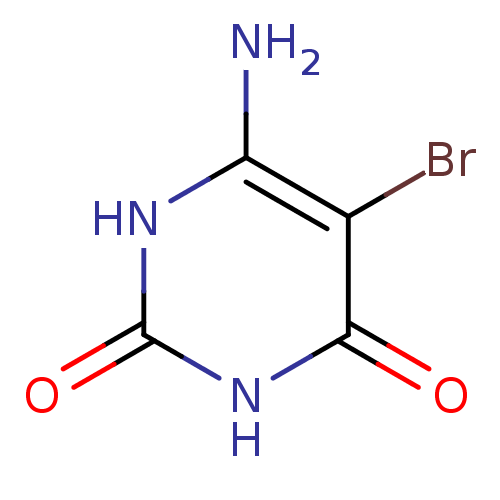
(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of human placental thymidine phosphorylase using [6-3H]dThd as substrate after 5 mins by scintillation counting analysis |
Eur J Med Chem 144: 41-51 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.016
BindingDB Entry DOI: 10.7270/Q24M9762 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50531739
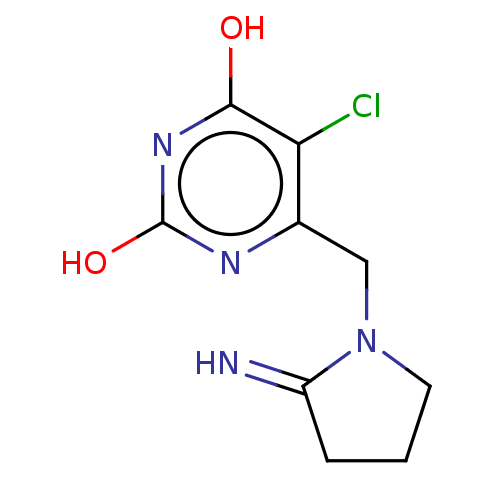
(MA-1 | TPI (freebase) | Tipiracil)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122771

(2-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122768
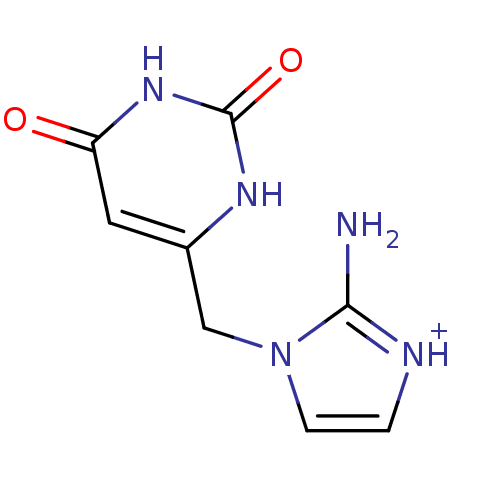
(2-Amino-1-(2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-...)Show InChI InChI=1S/C8H9N5O2/c9-7-10-1-2-13(7)4-5-3-6(14)12-8(15)11-5/h1-3H,4H2,(H2,9,10)(H2,11,12,14,15)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50531732
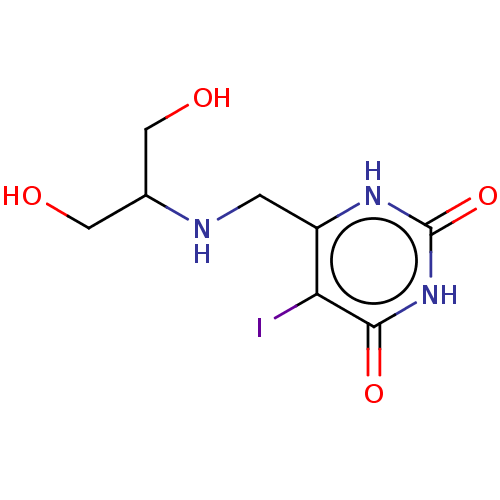
(CHEMBL4455140)Show InChI InChI=1S/C8H12IN3O4/c9-6-5(1-10-4(2-13)3-14)11-8(16)12-7(6)15/h4,10,13-14H,1-3H2,(H2,11,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50464651

(CHEMBL4293861)Show SMILES Cc1cc(=O)[nH]c(=O)n1\C(=N/O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C12H9Cl2N3O3/c1-6-4-10(18)15-12(19)17(6)11(16-20)7-2-3-8(13)9(14)5-7/h2-5,20H,1H3,(H,15,18,19)/b16-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) using thymidine as substrate after 1 hr by spectrophotometric analysis |
Eur J Med Chem 144: 41-51 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.016
BindingDB Entry DOI: 10.7270/Q24M9762 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50443422

(CHEMBL3087264)Show InChI InChI=1S/C6H7ClN4O2S/c7-3-2(1-14-5(8)9)10-6(13)11-4(3)12/h1H2,(H3,8,9)(H2,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human thymidine phosphorylase |
Eur J Med Chem 70: 400-10 (2013)
Article DOI: 10.1016/j.ejmech.2013.10.022
BindingDB Entry DOI: 10.7270/Q2CZ38NN |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122768

(2-Amino-1-(2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-...)Show InChI InChI=1S/C8H9N5O2/c9-7-10-1-2-13(7)4-5-3-6(14)12-8(15)11-5/h1-3H,4H2,(H2,9,10)(H2,11,12,14,15)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50531729
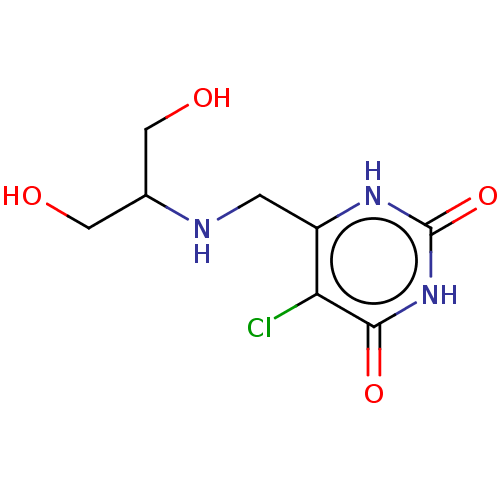
(CHEMBL4469338)Show InChI InChI=1S/C8H12ClN3O4/c9-6-5(1-10-4(2-13)3-14)11-8(16)12-7(6)15/h4,10,13-14H,1-3H2,(H2,11,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50531734

(CHEMBL4468378)Show InChI InChI=1S/C7H10IN3O3/c8-5-4(3-9-1-2-12)10-7(14)11-6(5)13/h9,12H,1-3H2,(H2,10,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50464648
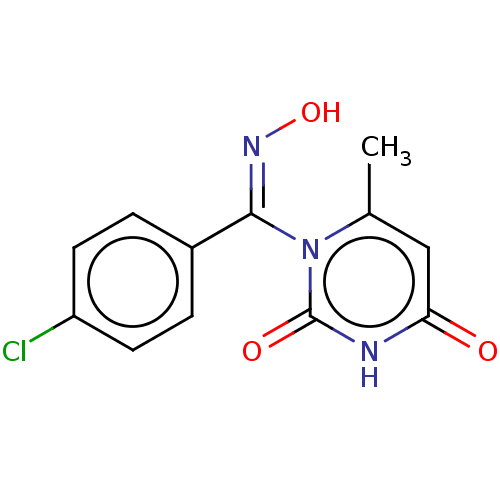
(CHEMBL4291353)Show InChI InChI=1S/C12H10ClN3O3/c1-7-6-10(17)14-12(18)16(7)11(15-19)8-2-4-9(13)5-3-8/h2-6,19H,1H3,(H,14,17,18)/b15-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) using thymidine as substrate after 1 hr by spectrophotometric analysis |
Eur J Med Chem 144: 41-51 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.016
BindingDB Entry DOI: 10.7270/Q24M9762 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122765

(6-(2-Nitro-imidazol-1-ylmethyl)-1H-pyrimidine-2,4-...)Show SMILES [O-][N+](=O)c1nccn1Cc1cc(=[OH+])[n-]c(=[OH+])[n-]1 Show InChI InChI=1S/C8H7N5O4/c14-6-3-5(10-7(15)11-6)4-12-2-1-9-8(12)13(16)17/h1-3H,4H2,(H2,10,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194179
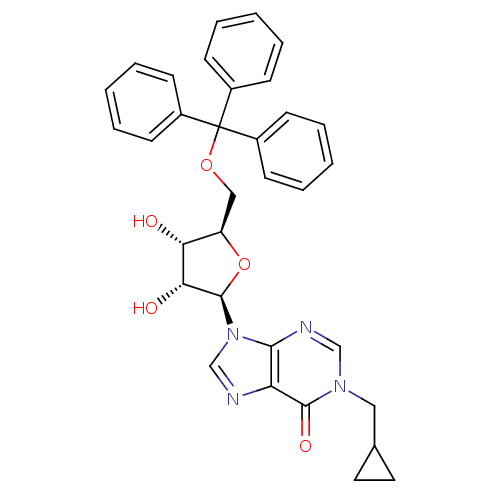
(1-(cyclopropyl)methyl-5'-O-tritylinosine | CHEMBL3...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(CC1CC1)c2=O Show InChI InChI=1S/C33H32N4O5/c38-28-26(42-32(29(28)39)37-21-34-27-30(37)35-20-36(31(27)40)18-22-16-17-22)19-41-33(23-10-4-1-5-11-23,24-12-6-2-7-13-24)25-14-8-3-9-15-25/h1-15,20-22,26,28-29,32,38-39H,16-19H2/t26-,28-,29-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50464643

(CHEMBL4290057)Show InChI InChI=1S/C12H10BrN3O3/c1-7-6-10(17)14-12(18)16(7)11(15-19)8-2-4-9(13)5-3-8/h2-6,19H,1H3,(H,14,17,18)/b15-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) using thymidine as substrate after 1 hr by spectrophotometric analysis |
Eur J Med Chem 144: 41-51 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.016
BindingDB Entry DOI: 10.7270/Q24M9762 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50439105

(CHEMBL2418061)Show InChI InChI=1S/C11H7Cl2N5OS/c12-6-2-1-5(3-7(6)13)4-8-14-9-15-10(20)16-11(19)18(9)17-8/h1-3H,4H2,(H2,14,15,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli using thymidine as substrate after 4 to 20 mins by spectrophoto... |
Eur J Med Chem 67: 325-34 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.051
BindingDB Entry DOI: 10.7270/Q2QR4ZJ0 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20052

(5-bromo-6-({2-[(5-bromo-2,6-dioxo-1,2,3,6-tetrahyd...)Show SMILES Brc1c(NCCNc2[nH]c(=O)[nH]c(=O)c2Br)[nH]c(=O)[nH]c1=O Show InChI InChI=1S/C10H10Br2N6O4/c11-3-5(15-9(21)17-7(3)19)13-1-2-14-6-4(12)8(20)18-10(22)16-6/h1-2H2,(H3,13,15,17,19,21)(H3,14,16,18,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 6.4 | 37 |
Academy of Sciences of the Czech Republic
| Assay Description
The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... |
Bioorg Med Chem Lett 16: 1335-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.050
BindingDB Entry DOI: 10.7270/Q2QR4VCH |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50464649
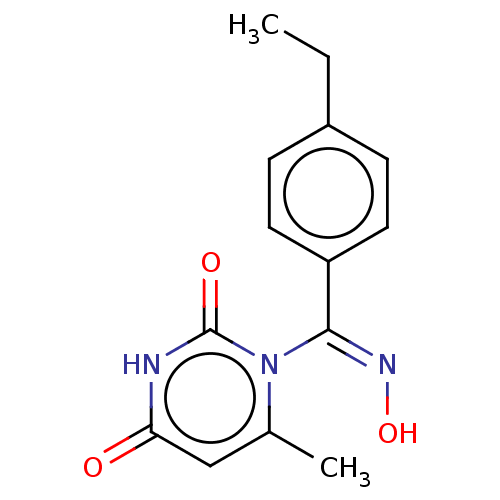
(CHEMBL4278709)Show InChI InChI=1S/C14H15N3O3/c1-3-10-4-6-11(7-5-10)13(16-20)17-9(2)8-12(18)15-14(17)19/h4-8,20H,3H2,1-2H3,(H,15,18,19)/b16-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan University
Curated by ChEMBL
| Assay Description
Inhibition of thymidine phosphorylase (unknown origin) using thymidine as substrate after 1 hr by spectrophotometric analysis |
Eur J Med Chem 144: 41-51 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.016
BindingDB Entry DOI: 10.7270/Q24M9762 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20034

(5-bromo-6-hydrazinyl-1,3-diazinane-2,4-dione | ura...)Show InChI InChI=1S/C4H5BrN4O2/c5-1-2(9-6)7-4(11)8-3(1)10/h6H2,(H3,7,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | 6.4 | 37 |
Academy of Sciences of the Czech Republic
| Assay Description
The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... |
Bioorg Med Chem Lett 16: 1335-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.050
BindingDB Entry DOI: 10.7270/Q2QR4VCH |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50004462

(CHEMBL3233329)Show SMILES NC(=S)Nc1nn2c([nH]c(=S)[nH]c2=O)c1Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6OS2/c15-14(16,17)7-3-1-6(2-4-7)5-8-9(19-11(18)25)22-23-10(8)20-12(26)21-13(23)24/h1-4H,5H2,(H3,18,19,22,25)(H2,20,21,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli assessed as conversion of thymidine to thymine after 4 to 20 mi... |
Eur J Med Chem 78: 294-303 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.063
BindingDB Entry DOI: 10.7270/Q2SF2XP4 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20051

(5-bromo-6-[4-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro...)Show SMILES Brc1c([nH]c(=O)[nH]c1=O)N1CCN(CC1)c1[nH]c(=O)[nH]c(=O)c1Br Show InChI InChI=1S/C12H12Br2N6O4/c13-5-7(15-11(23)17-9(5)21)19-1-2-20(4-3-19)8-6(14)10(22)18-12(24)16-8/h1-4H2,(H2,15,17,21,23)(H2,16,18,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 6.4 | 37 |
Academy of Sciences of the Czech Republic
| Assay Description
The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... |
Bioorg Med Chem Lett 16: 1335-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.050
BindingDB Entry DOI: 10.7270/Q2QR4VCH |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50004457
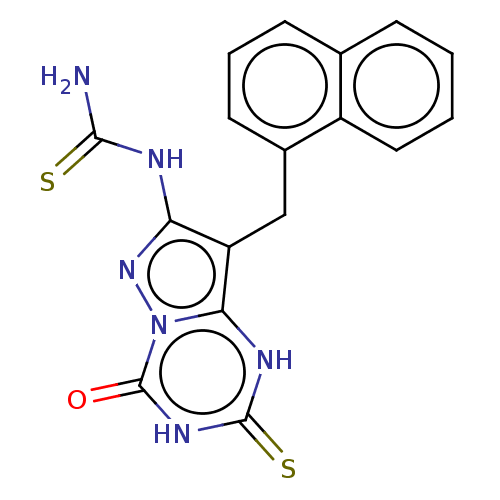
(CHEMBL3233340)Show SMILES NC(=S)Nc1nn2c([nH]c(=S)[nH]c2=O)c1Cc1cccc2ccccc12 Show InChI InChI=1S/C17H14N6OS2/c18-15(25)19-13-12(14-20-16(26)21-17(24)23(14)22-13)8-10-6-3-5-9-4-1-2-7-11(9)10/h1-7H,8H2,(H3,18,19,22,25)(H2,20,21,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli assessed as conversion of thymidine to thymine after 4 to 20 mi... |
Eur J Med Chem 78: 294-303 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.063
BindingDB Entry DOI: 10.7270/Q2SF2XP4 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
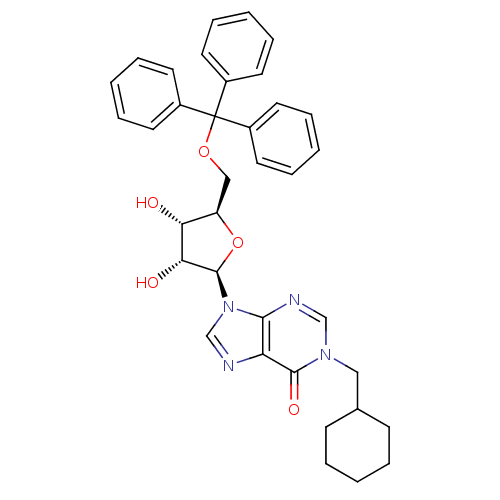
(Homo sapiens (Human)) | BDBM50194181
(1-(cyclohexyl)methyl-5'-O-tritylinosine | CHEMBL21...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(CC1CCCCC1)c2=O Show InChI InChI=1S/C36H38N4O5/c41-31-29(22-44-36(26-15-7-2-8-16-26,27-17-9-3-10-18-27)28-19-11-4-12-20-28)45-35(32(31)42)40-24-37-30-33(40)38-23-39(34(30)43)21-25-13-5-1-6-14-25/h2-4,7-12,15-20,23-25,29,31-32,35,41-42H,1,5-6,13-14,21-22H2/t29-,31-,32-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50004456

(CHEMBL3233339)Show SMILES NC(=S)Nc1nn2c([nH]c(=S)[nH]c2=O)c1Cc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C19H16N6O2S2/c20-17(28)21-15-14(16-22-18(29)23-19(26)25(16)24-15)10-11-6-8-13(9-7-11)27-12-4-2-1-3-5-12/h1-9H,10H2,(H3,20,21,24,28)(H2,22,23,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli assessed as conversion of thymidine to thymine after 4 to 20 mi... |
Eur J Med Chem 78: 294-303 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.063
BindingDB Entry DOI: 10.7270/Q2SF2XP4 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
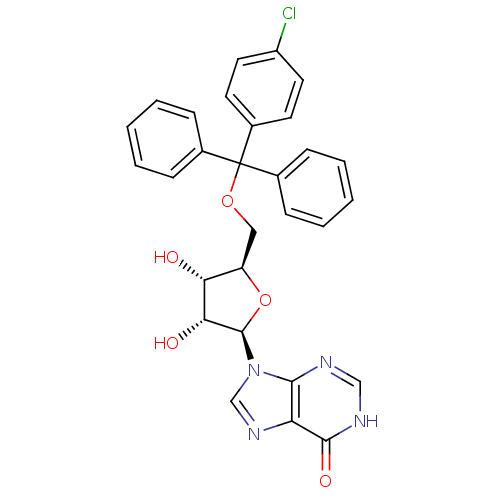
(Homo sapiens (Human)) | BDBM50194173
(5'-O-[(4-chlorophenyl)-1,1-(diphenyl)methyl]inosin...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccc(Cl)cc2)O[C@H]([C@@H]1O)n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C29H25ClN4O5/c30-21-13-11-20(12-14-21)29(18-7-3-1-4-8-18,19-9-5-2-6-10-19)38-15-22-24(35)25(36)28(39-22)34-17-33-23-26(34)31-16-32-27(23)37/h1-14,16-17,22,24-25,28,35-36H,15H2,(H,31,32,37)/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
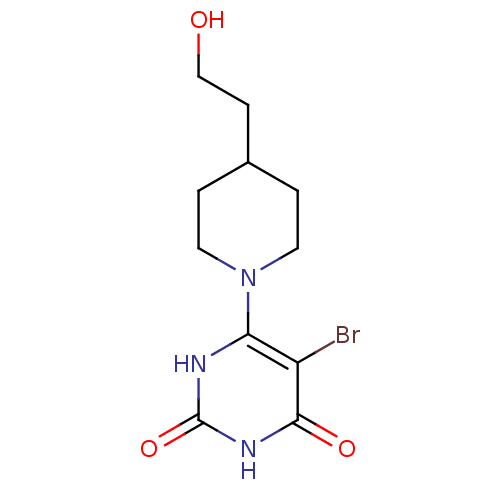
(Homo sapiens (Human)) | BDBM20040
(5-bromo-6-[4-(2-hydroxyethyl)piperidin-1-yl]-1,3-d...)Show InChI InChI=1S/C11H16BrN3O3/c12-8-9(13-11(18)14-10(8)17)15-4-1-7(2-5-15)3-6-16/h7,16H,1-6H2,(H2,13,14,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 6.4 | 37 |
Academy of Sciences of the Czech Republic
| Assay Description
The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... |
Bioorg Med Chem Lett 16: 1335-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.050
BindingDB Entry DOI: 10.7270/Q2QR4VCH |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20032

(5-bromo-6-(pyrrolidin-1-yl)-1,3-diazinane-2,4-dion...)Show InChI InChI=1S/C8H10BrN3O2/c9-5-6(12-3-1-2-4-12)10-8(14)11-7(5)13/h1-4H2,(H2,10,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | 6.4 | 37 |
Academy of Sciences of the Czech Republic
| Assay Description
The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... |
Bioorg Med Chem Lett 16: 1335-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.050
BindingDB Entry DOI: 10.7270/Q2QR4VCH |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20038

(5-bromo-6-[3-(hydroxymethyl)piperidin-1-yl]-1,3-di...)Show InChI InChI=1S/C10H14BrN3O3/c11-7-8(12-10(17)13-9(7)16)14-3-1-2-6(4-14)5-15/h6,15H,1-5H2,(H2,12,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 6.4 | 37 |
Academy of Sciences of the Czech Republic
| Assay Description
The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... |
Bioorg Med Chem Lett 16: 1335-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.050
BindingDB Entry DOI: 10.7270/Q2QR4VCH |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50531742
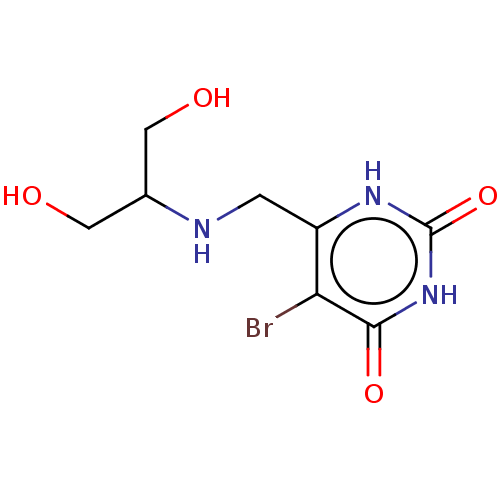
(CHEMBL4579188)Show InChI InChI=1S/C8H12BrN3O4/c9-6-5(1-10-4(2-13)3-14)11-8(16)12-7(6)15/h4,10,13-14H,1-3H2,(H2,11,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... |
J Med Chem 62: 1231-1245 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01305
BindingDB Entry DOI: 10.7270/Q2FT8QHC |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50004455
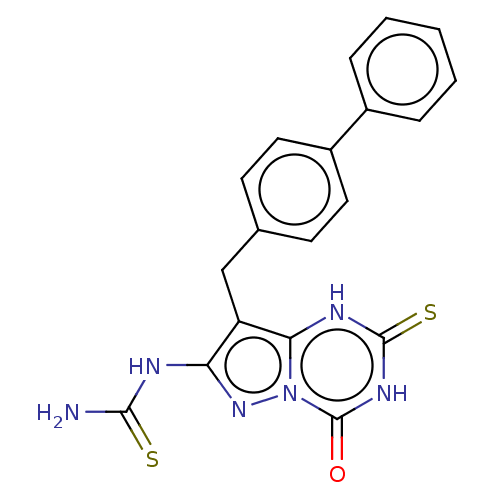
(CHEMBL3233338)Show SMILES NC(=S)Nc1nn2c([nH]c(=S)[nH]c2=O)c1Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C19H16N6OS2/c20-17(27)21-15-14(16-22-18(28)23-19(26)25(16)24-15)10-11-6-8-13(9-7-11)12-4-2-1-3-5-12/h1-9H,10H2,(H3,20,21,24,27)(H2,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli assessed as conversion of thymidine to thymine after 4 to 20 mi... |
Eur J Med Chem 78: 294-303 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.063
BindingDB Entry DOI: 10.7270/Q2SF2XP4 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data