 Found 211 hits of ki for UniProtKB: P08684
Found 211 hits of ki for UniProtKB: P08684 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50351401

(CHEMBL1819091)Show SMILES CC[C@@]1(N)CCCN(C1)c1cc2n(C)c(=O)n(C)c(=O)c2n1Cc1cc(F)ccc1C#N |r| Show InChI InChI=1S/C23H27FN6O2/c1-4-23(26)8-5-9-29(14-23)19-11-18-20(21(31)28(3)22(32)27(18)2)30(19)13-16-10-17(24)7-6-15(16)12-25/h6-7,10-11H,4-5,8-9,13-14,26H2,1-3H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis |
Bioorg Med Chem 19: 5490-9 (2011)
Article DOI: 10.1016/j.bmc.2011.07.042
BindingDB Entry DOI: 10.7270/Q2T72HT6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50351399
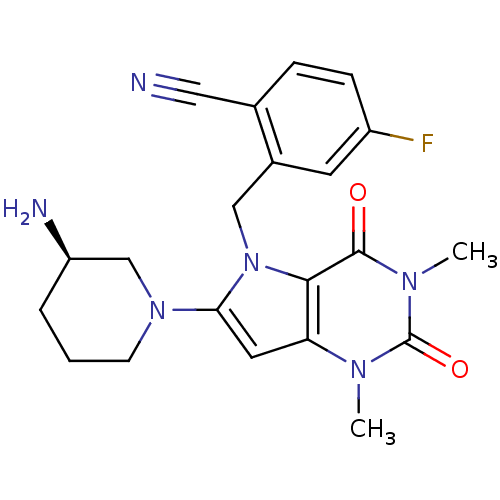
(CHEMBL1819089)Show SMILES Cn1c2cc(N3CCC[C@@H](N)C3)n(Cc3cc(F)ccc3C#N)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H23FN6O2/c1-25-17-9-18(27-7-3-4-16(24)12-27)28(19(17)20(29)26(2)21(25)30)11-14-8-15(22)6-5-13(14)10-23/h5-6,8-9,16H,3-4,7,11-12,24H2,1-2H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis |
Bioorg Med Chem 19: 5490-9 (2011)
Article DOI: 10.1016/j.bmc.2011.07.042
BindingDB Entry DOI: 10.7270/Q2T72HT6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50267297

(CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...)Show SMILES COC(=O)N[C@H](C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2ccccc2)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C47H60N6O6/c1-46(2,3)40(51-44(57)59-7)42(55)49-36(28-33-21-23-35(24-22-33)37-20-14-15-25-48-37)30-39(54)38(29-32-16-10-8-11-17-32)50-43(56)41(47(4,5)6)53-27-26-52(45(53)58)31-34-18-12-9-13-19-34/h8-25,36,38-41,54H,26-31H2,1-7H3,(H,49,55)(H,50,56)(H,51,57)/t36-,38-,39-,40+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044424
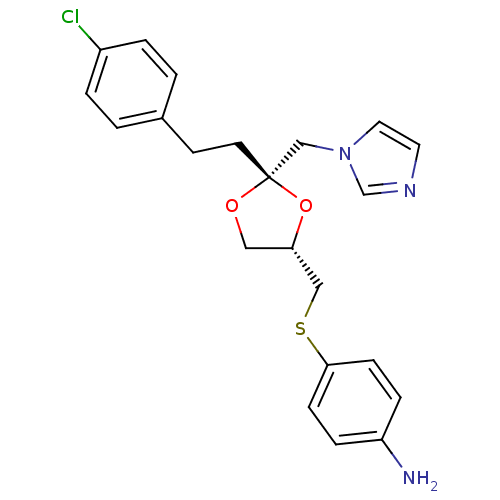
(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314498
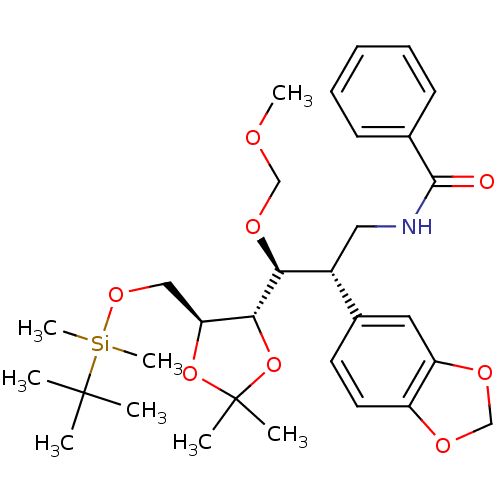
(CHEMBL1089299 | N-((2S,3S)-2-(benzo[d][1,3]dioxol-...)Show SMILES COCO[C@@H]([C@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C31H45NO8Si/c1-30(2,3)41(7,8)38-18-26-28(40-31(4,5)39-26)27(37-19-34-6)23(17-32-29(33)21-12-10-9-11-13-21)22-14-15-24-25(16-22)36-20-35-24/h9-16,23,26-28H,17-20H2,1-8H3,(H,32,33)/t23-,26+,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... |
Bioorg Med Chem Lett 20: 2335-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.157
BindingDB Entry DOI: 10.7270/Q21V5F4M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300539
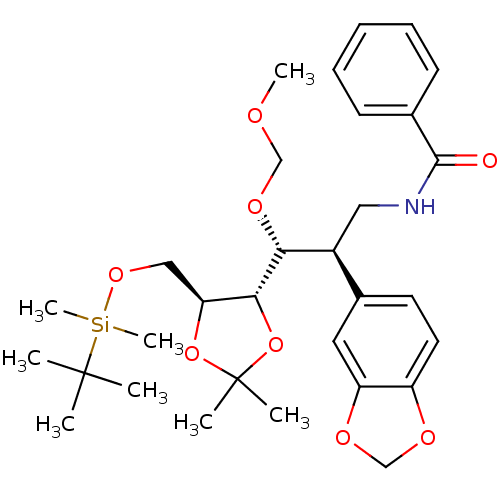
(CHEMBL573430 | N-((2R,3R)-2-(benzo[d][1,3]dioxol-5...)Show SMILES COCO[C@H]([C@@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C31H45NO8Si/c1-30(2,3)41(7,8)38-18-26-28(40-31(4,5)39-26)27(37-19-34-6)23(17-32-29(33)21-12-10-9-11-13-21)22-14-15-24-25(16-22)36-20-35-24/h9-16,23,26-28H,17-20H2,1-8H3,(H,32,33)/t23-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50259874
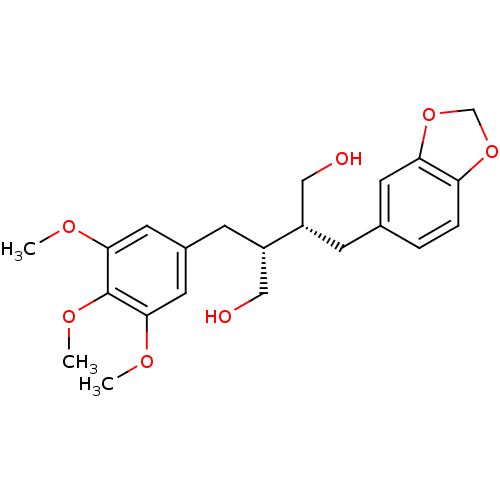
((-)-dihydroclusin | CHEMBL469916)Show SMILES COc1cc(C[C@@H](CO)[C@H](CO)Cc2ccc3OCOc3c2)cc(OC)c1OC |r| Show InChI InChI=1S/C22H28O7/c1-25-20-9-15(10-21(26-2)22(20)27-3)7-17(12-24)16(11-23)6-14-4-5-18-19(8-14)29-13-28-18/h4-5,8-10,16-17,23-24H,6-7,11-13H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50267295

(CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N1CCN(Cc2cccc(n2)C(C)(C)O)C1=O)C(C)(C)C)Cc1ccc(cc1)-c1ccccn1)C(C)(C)C |r| Show InChI InChI=1S/C49H65N7O7/c1-47(2,3)41(54-45(60)63-9)43(58)52-36(28-33-21-23-34(24-22-33)37-19-13-14-25-50-37)30-39(57)38(29-32-16-11-10-12-17-32)53-44(59)42(48(4,5)6)56-27-26-55(46(56)61)31-35-18-15-20-40(51-35)49(7,8)62/h10-25,36,38-39,41-42,57,62H,26-31H2,1-9H3,(H,52,58)(H,53,59)(H,54,60)/t36-,38+,39+,41-,42-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation |
J Med Chem 52: 2571-86 (2009)
Article DOI: 10.1021/jm900044w
BindingDB Entry DOI: 10.7270/Q2G160Q0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300543
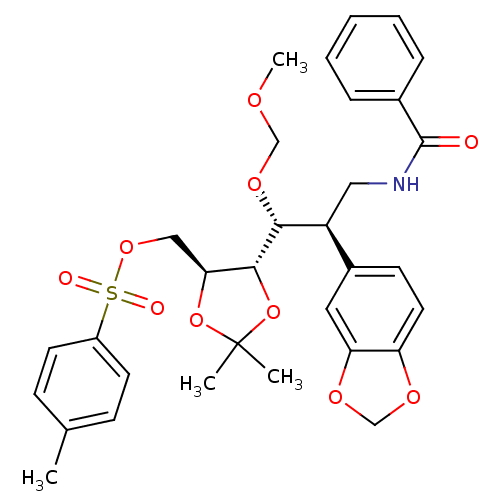
(((4S,5S)-5-((1R,2R)-3-benzamido-2-(benzo[d][1,3]di...)Show SMILES COCO[C@H]([C@@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1COS(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C32H37NO10S/c1-21-10-13-24(14-11-21)44(35,36)41-18-28-30(43-32(2,3)42-28)29(40-19-37-4)25(17-33-31(34)22-8-6-5-7-9-22)23-12-15-26-27(16-23)39-20-38-26/h5-16,25,28-30H,17-20H2,1-4H3,(H,33,34)/t25-,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314501
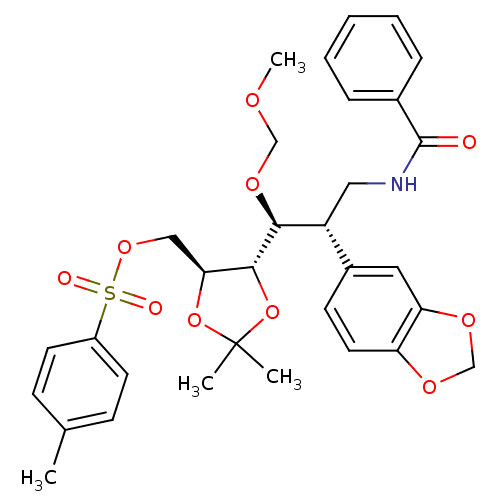
(((4S,5S)-5-((1S,2S)-3-benzamido-2-(benzo[d][1,3]di...)Show SMILES COCO[C@@H]([C@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1COS(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C32H37NO10S/c1-21-10-13-24(14-11-21)44(35,36)41-18-28-30(43-32(2,3)42-28)29(40-19-37-4)25(17-33-31(34)22-8-6-5-7-9-22)23-12-15-26-27(16-23)39-20-38-26/h5-16,25,28-30H,17-20H2,1-4H3,(H,33,34)/t25-,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... |
Bioorg Med Chem Lett 20: 2335-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.157
BindingDB Entry DOI: 10.7270/Q21V5F4M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50106864
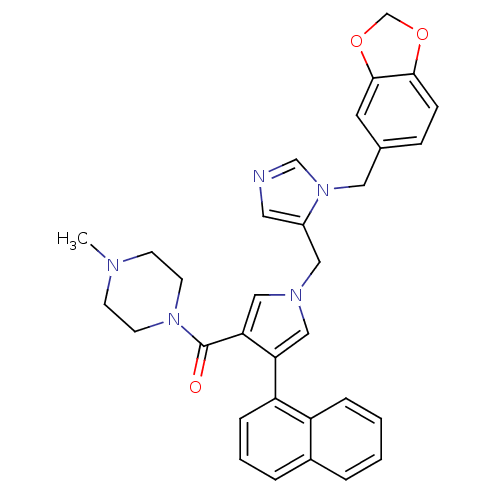
((1-((1-(benzo[d][1,3]dioxol-5-ylmethyl)-1H-imidazo...)Show SMILES CN1CCN(CC1)C(=O)c1cn(Cc2cncn2Cc2ccc3OCOc3c2)cc1-c1cccc2ccccc12 Show InChI InChI=1S/C32H31N5O3/c1-34-11-13-36(14-12-34)32(38)29-20-35(19-28(29)27-8-4-6-24-5-2-3-7-26(24)27)18-25-16-33-21-37(25)17-23-9-10-30-31(15-23)40-22-39-30/h2-10,15-16,19-21H,11-14,17-18,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences R&D Center
Curated by ChEMBL
| Assay Description
Reversible inhibition of human CYP3A4 in liver microsomes by Dixon and Cornish-Bowden plot analysis |
Bioorg Med Chem Lett 22: 3067-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.070
BindingDB Entry DOI: 10.7270/Q26974MZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50259867
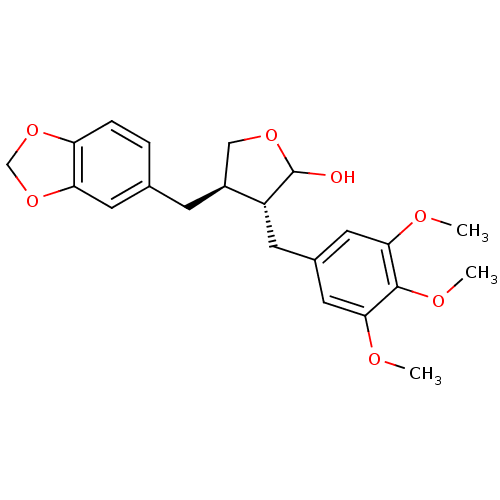
((-)-clusin | CHEMBL479701)Show SMILES COc1cc(C[C@@H]2[C@@H](Cc3ccc4OCOc4c3)COC2O)cc(OC)c1OC |r| Show InChI InChI=1S/C22H26O7/c1-24-19-9-14(10-20(25-2)21(19)26-3)7-16-15(11-27-22(16)23)6-13-4-5-17-18(8-13)29-12-28-17/h4-5,8-10,15-16,22-23H,6-7,11-12H2,1-3H3/t15-,16+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50519563
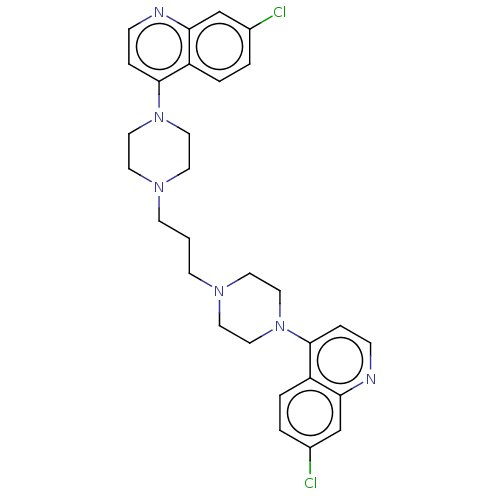
(Piperaquine)Show SMILES Clc1ccc2c(ccnc2c1)N1CCN(CCCN2CCN(CC2)c2ccnc3cc(Cl)ccc23)CC1 Show InChI InChI=1S/C29H32Cl2N6/c30-22-2-4-24-26(20-22)32-8-6-28(24)36-16-12-34(13-17-36)10-1-11-35-14-18-37(19-15-35)29-7-9-33-27-21-23(31)3-5-25(27)29/h2-9,20-21H,1,10-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 62: 10526-10562 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00761
BindingDB Entry DOI: 10.7270/Q21J9F41 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4-mediated N-demethylation in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088503
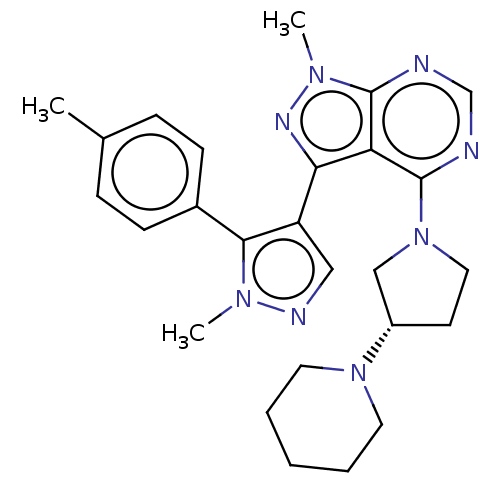
(CHEMBL3527048)Show SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12 |r| Show InChI InChI=1S/C26H32N8/c1-18-7-9-19(10-8-18)24-21(15-29-31(24)2)23-22-25(32(3)30-23)27-17-28-26(22)34-14-11-20(16-34)33-12-5-4-6-13-33/h7-10,15,17,20H,4-6,11-14,16H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A4 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... |
Drug Metab Dispos 40: 1686-97 (2012)
Article DOI: 10.1124/dmd.112.045302
BindingDB Entry DOI: 10.7270/Q2GT5PW2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50418094
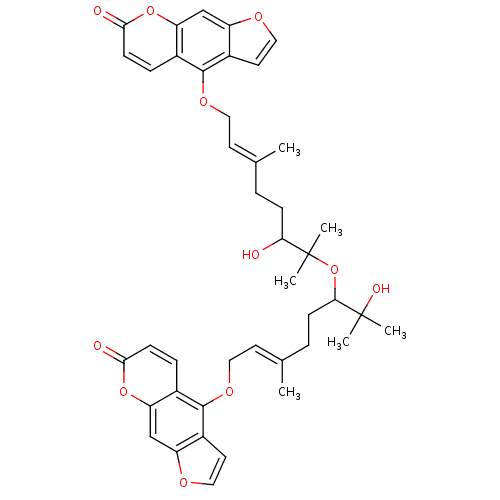
(CHEMBL1743357)Show SMILES C\C(CCC(O)C(C)(C)OC(CC\C(C)=C\COc1c2ccoc2cc2oc(=O)ccc12)C(C)(C)O)=C/COc1c2ccoc2cc2oc(=O)ccc12 Show InChI InChI=1S/C42H46O11/c1-25(15-19-49-39-27-9-13-37(44)51-33(27)23-31-29(39)17-21-47-31)7-11-35(43)42(5,6)53-36(41(3,4)46)12-8-26(2)16-20-50-40-28-10-14-38(45)52-34(28)24-32-30(40)18-22-48-32/h9-10,13-18,21-24,35-36,43,46H,7-8,11-12,19-20H2,1-6H3/b25-15+,26-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by nifedipine oxidation, omeprazole 3-hydroxylation and omeprazole sulfoxydation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50241937
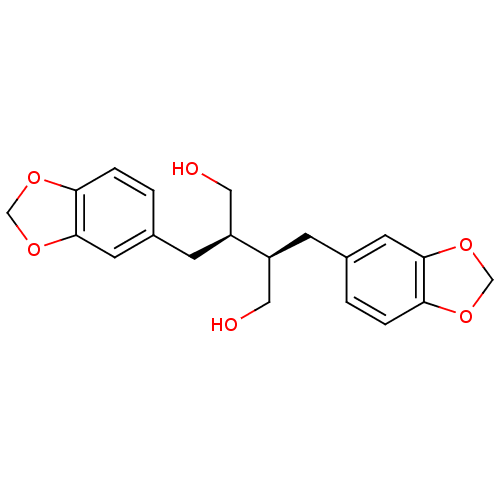
((-)-dihydrocubebin | CHEMBL486597 | dihydrocubebin)Show SMILES OC[C@H](Cc1ccc2OCOc2c1)[C@H](CO)Cc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C20H22O6/c21-9-15(5-13-1-3-17-19(7-13)25-11-23-17)16(10-22)6-14-2-4-18-20(8-14)26-12-24-18/h1-4,7-8,15-16,21-22H,5-6,9-12H2/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50388532
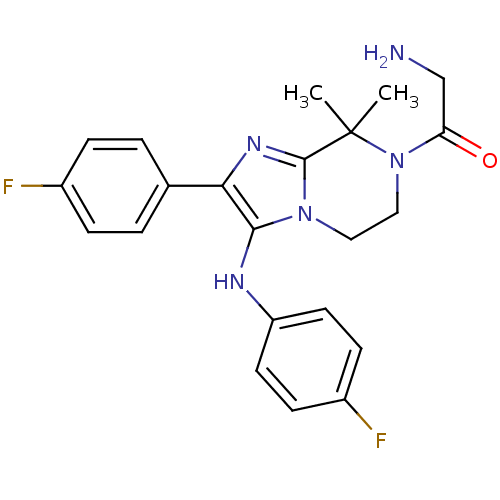
(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 62: 10526-10562 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00761
BindingDB Entry DOI: 10.7270/Q21J9F41 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300538

(CHEMBL574914 | N-((2R,3R)-2-(benzo[d][1,3]dioxol-5...)Show SMILES CC(C)(C)[Si](C)(C)OC[C@@H]1OC(C)(C)O[C@H]1[C@H](O)[C@@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C29H41NO7Si/c1-28(2,3)38(6,7)35-17-24-26(37-29(4,5)36-24)25(31)21(16-30-27(32)19-11-9-8-10-12-19)20-13-14-22-23(15-20)34-18-33-22/h8-15,21,24-26,31H,16-18H2,1-7H3,(H,30,32)/t21-,24-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088503

(CHEMBL3527048)Show SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12 |r| Show InChI InChI=1S/C26H32N8/c1-18-7-9-19(10-8-18)24-21(15-29-31(24)2)23-22-25(32(3)30-23)27-17-28-26(22)34-14-11-20(16-34)33-12-5-4-6-13-33/h7-10,15,17,20H,4-6,11-14,16H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A4 in presence of human P450 oxidoreductase and b5 assessed as decreas... |
Drug Metab Dispos 40: 1686-97 (2012)
Article DOI: 10.1124/dmd.112.045302
BindingDB Entry DOI: 10.7270/Q2GT5PW2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50241524
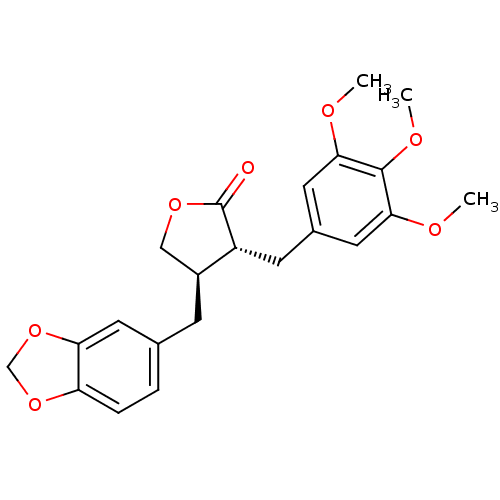
((-)-deoxypodorhizone | (-)-yatein | CHEMBL471067)Show SMILES COc1cc(C[C@@H]2[C@@H](Cc3ccc4OCOc4c3)COC2=O)cc(OC)c1OC |r| Show InChI InChI=1S/C22H24O7/c1-24-19-9-14(10-20(25-2)21(19)26-3)7-16-15(11-27-22(16)23)6-13-4-5-17-18(8-13)29-12-28-17/h4-5,8-10,15-16H,6-7,11-12H2,1-3H3/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM20607
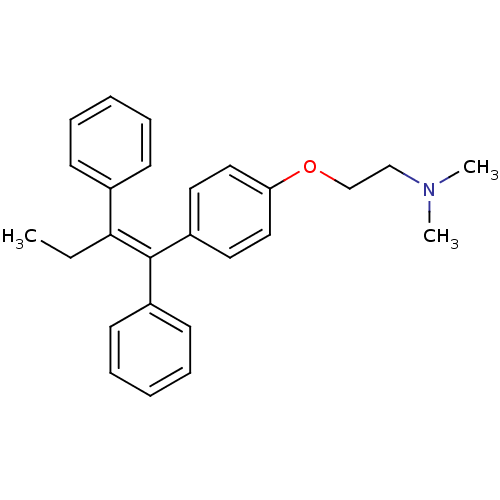
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50293601
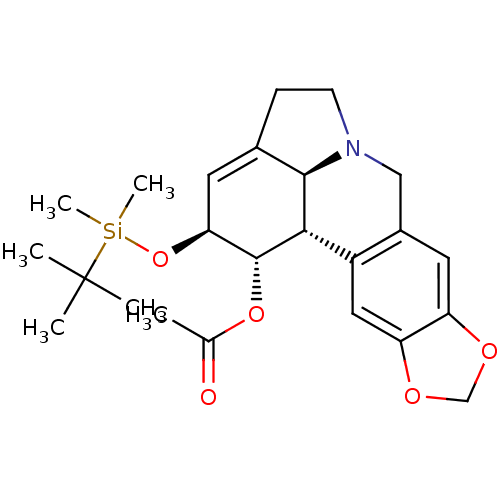
((1S,2S,3a1S,12bS)-2-(tert-butyldimethylsilyloxy)-2...)Show SMILES CC(=O)O[C@@H]1[C@@H](O[Si](C)(C)C(C)(C)C)C=C2CCN3Cc4cc5OCOc5cc4[C@H]1[C@@H]23 |r,t:14| Show InChI InChI=1S/C24H33NO5Si/c1-14(26)29-23-20(30-31(5,6)24(2,3)4)9-15-7-8-25-12-16-10-18-19(28-13-27-18)11-17(16)21(23)22(15)25/h9-11,20-23H,7-8,12-13H2,1-6H3/t20-,21-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 3233-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.086
BindingDB Entry DOI: 10.7270/Q2X92BBG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50418095
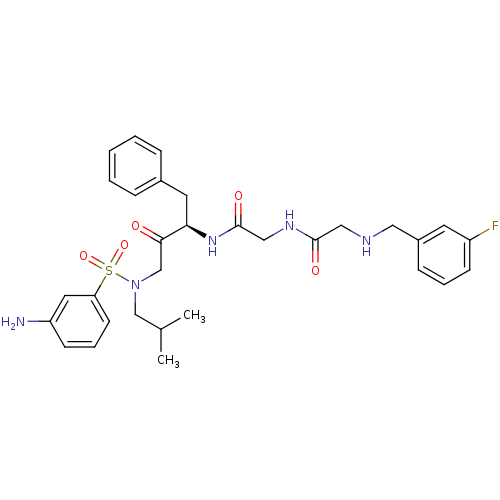
(CHEMBL1743352)Show SMILES CC(C)CN(CC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNCc1cccc(F)c1)S(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C31H38FN5O5S/c1-22(2)20-37(43(41,42)27-13-7-12-26(33)16-27)21-29(38)28(15-23-8-4-3-5-9-23)36-31(40)19-35-30(39)18-34-17-24-10-6-11-25(32)14-24/h3-14,16,22,28,34H,15,17-21,33H2,1-2H3,(H,35,39)(H,36,40)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50314499

(CHEMBL1089300 | tert-butyl(2S,3S)-2-(benzo[d][1,3]...)Show SMILES COCO[C@@H]([C@H](CNC(=O)OC(C)(C)C)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C29H49NO9Si/c1-27(2,3)39-26(31)30-15-20(19-12-13-21-22(14-19)34-18-33-21)24(35-17-32-9)25-23(37-29(7,8)38-25)16-36-40(10,11)28(4,5)6/h12-14,20,23-25H,15-18H2,1-11H3,(H,30,31)/t20-,23+,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... |
Bioorg Med Chem Lett 20: 2335-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.157
BindingDB Entry DOI: 10.7270/Q21V5F4M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50584760
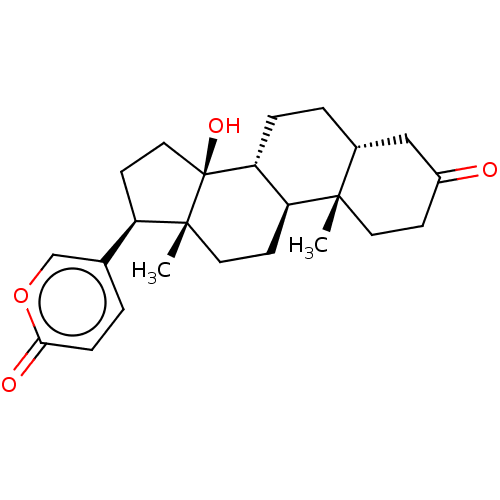
(CHEMBL2068968)Show SMILES [H][C@]12CC[C@]3([H])[C@]([H])(CC[C@]4(C)[C@H](CC[C@]34O)c3ccc(=O)oc3)[C@@]1(C)CCC(=O)C2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inactivation of recombinant human CYP3A4 assessed as inhibition constant using midazolam as substrate preincubated for 5 mins followed by beta-NADP a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01875
BindingDB Entry DOI: 10.7270/Q2PZ5DQR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50418096
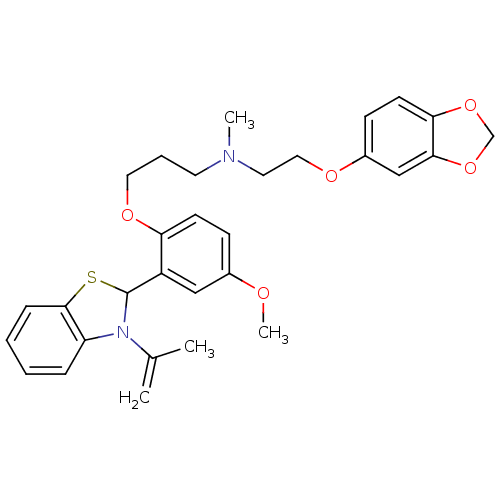
(CHEMBL1743364)Show SMILES COc1ccc(OCCCN(C)CCOc2ccc3OCOc3c2)c(c1)C1Sc2ccccc2N1C(C)=C Show InChI InChI=1S/C30H34N2O5S/c1-21(2)32-25-8-5-6-9-29(25)38-30(32)24-18-22(33-4)10-12-26(24)35-16-7-14-31(3)15-17-34-23-11-13-27-28(19-23)37-20-36-27/h5-6,8-13,18-19,30H,1,7,14-17,20H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50293600
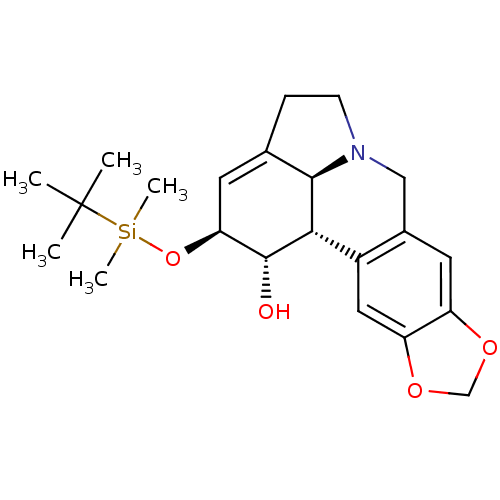
((1S,2S,3a1S,12bS)-2-(tert-butyldimethylsilyloxy)-2...)Show SMILES CC(C)(C)[Si](C)(C)O[C@H]1C=C2CCN3Cc4cc5OCOc5cc4[C@@H]([C@@H]23)[C@@H]1O |r,t:9| Show InChI InChI=1S/C22H31NO4Si/c1-22(2,3)28(4,5)27-18-8-13-6-7-23-11-14-9-16-17(26-12-25-16)10-15(14)19(20(13)23)21(18)24/h8-10,18-21,24H,6-7,11-12H2,1-5H3/t18-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 3233-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.086
BindingDB Entry DOI: 10.7270/Q2X92BBG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50117922
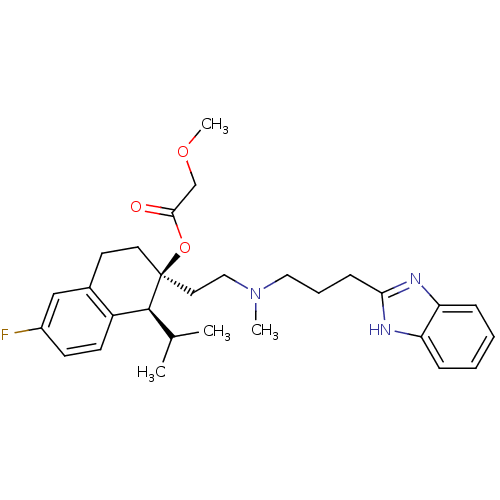
((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...)Show SMILES COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C |r| Show InChI InChI=1S/C29H38FN3O3/c1-20(2)28-23-12-11-22(30)18-21(23)13-14-29(28,36-27(34)19-35-4)15-17-33(3)16-7-10-26-31-24-8-5-6-9-25(24)32-26/h5-6,8-9,11-12,18,20,28H,7,10,13-17,19H2,1-4H3,(H,31,32)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 (unknown origin) expressed in baculosomes in presence of 1 mM NADPH |
Drug Metab Dispos 39: 1188-95 (2011)
Article DOI: 10.1124/dmd.111.038505
BindingDB Entry DOI: 10.7270/Q29888QX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP3A4 in human liver microsomes |
Drug Metab Dispos 40: 655-61 (2012)
Article DOI: 10.1124/dmd.111.043018
BindingDB Entry DOI: 10.7270/Q22Z1796 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50418093
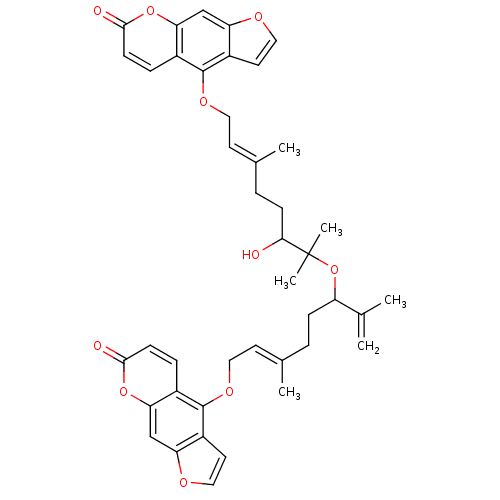
(CHEMBL1743356)Show SMILES C\C(CCC(OC(C)(C)C(O)CC\C(C)=C\COc1c2ccoc2cc2oc(=O)ccc12)C(C)=C)=C/COc1c2ccoc2cc2oc(=O)ccc12 Show InChI InChI=1S/C42H44O10/c1-25(2)32(11-7-26(3)15-19-48-40-28-9-13-38(44)50-35(28)23-33-30(40)17-21-46-33)52-42(5,6)37(43)12-8-27(4)16-20-49-41-29-10-14-39(45)51-36(29)24-34-31(41)18-22-47-34/h9-10,13-18,21-24,32,37,43H,1,7-8,11-12,19-20H2,2-6H3/b26-15+,27-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by nifedipine oxidation, omeprazole 3-hydroxylation and omeprazole sulfoxydation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50300541

(CHEMBL583953 | tert-butyl(2R,3R)-2-(benzo[d][1,3]d...)Show SMILES COCO[C@H]([C@@H](CNC(=O)OC(C)(C)C)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C29H49NO9Si/c1-27(2,3)39-26(31)30-15-20(19-12-13-21-22(14-19)34-18-33-21)24(35-17-32-9)25-23(37-29(7,8)38-25)16-36-40(10,11)28(4,5)6/h12-14,20,23-25H,15-18H2,1-11H3,(H,30,31)/t20-,23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc... |
Bioorg Med Chem Lett 19: 5607-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.032
BindingDB Entry DOI: 10.7270/Q2CF9Q4D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4-mediated testosterone-6-beta hydroxylation in human liver microsome |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50218812
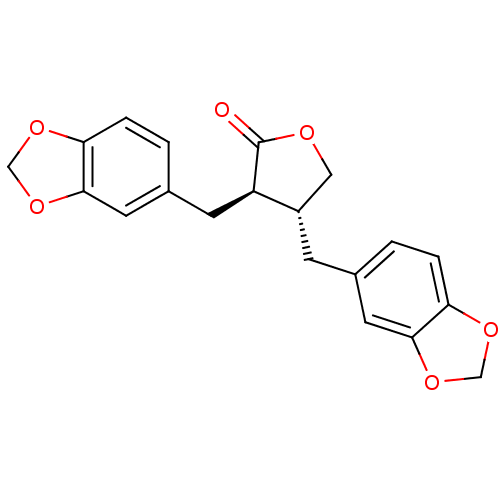
((3R,4R)-3,4-bis(1,3-benzodioxol-5-ylmethyl)dihydro...)Show SMILES O=C1OC[C@H](Cc2ccc3OCOc3c2)[C@H]1Cc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C20H18O6/c21-20-15(6-13-2-4-17-19(8-13)26-11-24-17)14(9-22-20)5-12-1-3-16-18(7-12)25-10-23-16/h1-4,7-8,14-15H,5-6,9-11H2/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50435005

(CHEMBL2386285)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN)cc1)c1ccccc1 Show InChI InChI=1S/C24H25NO2/c1-2-23(18-6-4-3-5-7-18)24(19-8-12-21(26)13-9-19)20-10-14-22(15-11-20)27-17-16-25/h3-15,26H,2,16-17,25H2,1H3/b24-23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay |
J Med Chem 58: 2623-48 (2015)
Article DOI: 10.1021/jm501218e
BindingDB Entry DOI: 10.7270/Q2TX3H32 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50418091
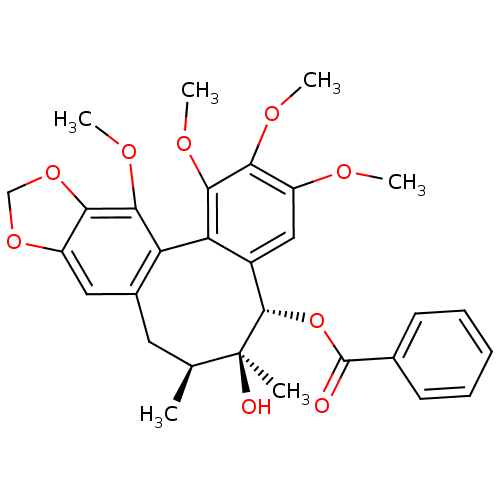
(CHEMBL404875)Show SMILES COc1cc2[C@H](OC(=O)c3ccccc3)[C@@](C)(O)[C@@H](C)Cc3cc4OCOc4c(OC)c3-c2c(OC)c1OC |r| Show InChI InChI=1S/C30H32O9/c1-16-12-18-13-21-25(38-15-37-21)26(35-5)22(18)23-19(14-20(33-3)24(34-4)27(23)36-6)28(30(16,2)32)39-29(31)17-10-8-7-9-11-17/h7-11,13-14,16,28,32H,12,15H2,1-6H3/t16-,28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| PubMed
| 399 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by N-demethylation of erythromycin |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50061306

((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50393263
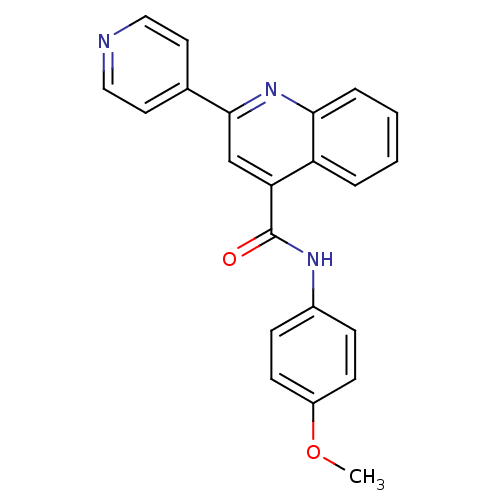
(CHEMBL2152014)Show SMILES COc1ccc(NC(=O)c2cc(nc3ccccc23)-c2ccncc2)cc1 Show InChI InChI=1S/C22H17N3O2/c1-27-17-8-6-16(7-9-17)24-22(26)19-14-21(15-10-12-23-13-11-15)25-20-5-3-2-4-18(19)20/h2-14H,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP3A4 NF-14 expressed in Escherichia coli assessed as formation of 6-beta-hydroxytestosterone using testosterone as substr... |
J Med Chem 55: 280-90 (2012)
Article DOI: 10.1021/jm201207h
BindingDB Entry DOI: 10.7270/Q2251K9D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data