 Found 7319 hits of ic50 data for polymerid = 2262,2300,2321,50000123,50001226,50001227,50001411,50002550,50002683
Found 7319 hits of ic50 data for polymerid = 2262,2300,2321,50000123,50001226,50001227,50001411,50002550,50002683 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272095
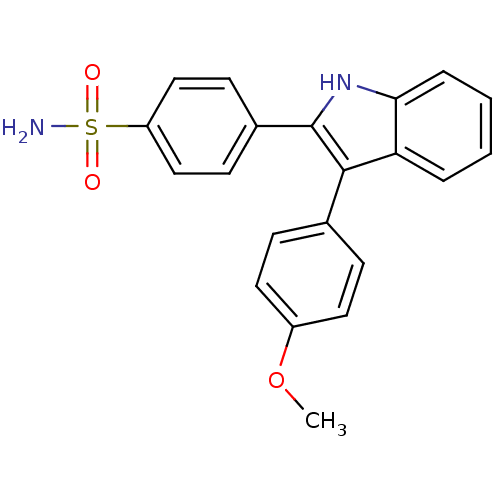
(4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-16-10-6-14(7-11-16)20-18-4-2-3-5-19(18)23-21(20)15-8-12-17(13-9-15)27(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272095

(4-(3-(4-methoxyphenyl)-1H-indol-2-yl)benzenesulfon...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O3S/c1-26-16-10-6-14(7-11-16)20-18-4-2-3-5-19(18)23-21(20)15-8-12-17(13-9-15)27(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272129
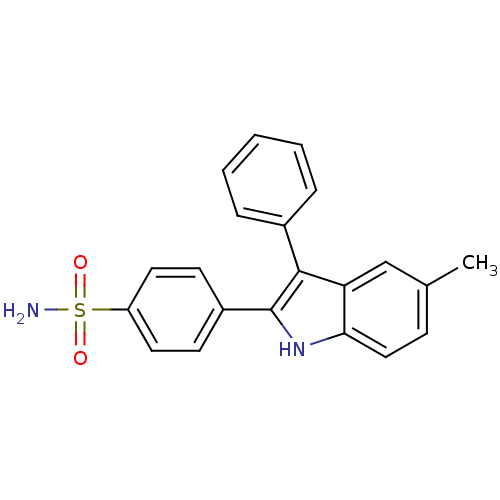
(4-(5-methyl-3-phenyl-1H-indol-2-yl)benzenesulfonam...)Show SMILES Cc1ccc2[nH]c(c(-c3ccccc3)c2c1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O2S/c1-14-7-12-19-18(13-14)20(15-5-3-2-4-6-15)21(23-19)16-8-10-17(11-9-16)26(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272131

(3-(4-fluorophenyl)-2-(4-(methylsulfonyl)phenyl)-1H...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(F)cc1 Show InChI InChI=1S/C21H16FNO2S/c1-26(24,25)17-12-8-15(9-13-17)21-20(14-6-10-16(22)11-7-14)18-4-2-3-5-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272096
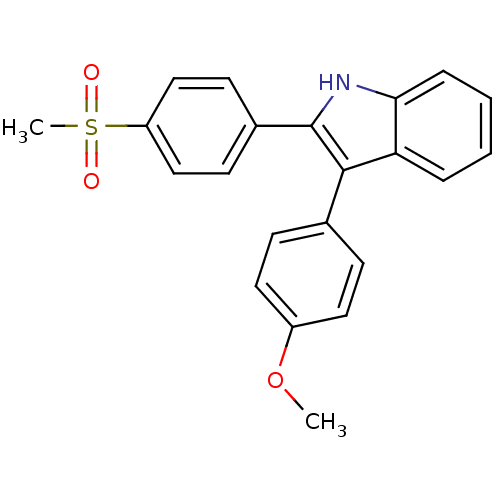
(3-(4-methoxyphenyl)-2-(4-(methylsulfonyl)phenyl)-1...)Show SMILES COc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO3S/c1-26-17-11-7-15(8-12-17)21-19-5-3-4-6-20(19)23-22(21)16-9-13-18(14-10-16)27(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272125
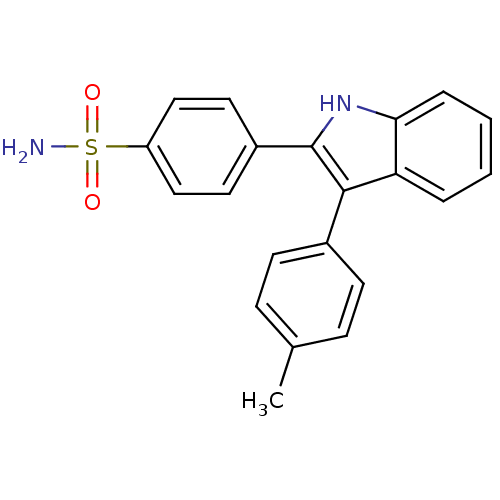
(4-(3-p-tolyl-1H-indol-2-yl)benzenesulfonamide | CH...)Show SMILES Cc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H18N2O2S/c1-14-6-8-15(9-7-14)20-18-4-2-3-5-19(18)23-21(20)16-10-12-17(13-11-16)26(22,24)25/h2-13,23H,1H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50094539
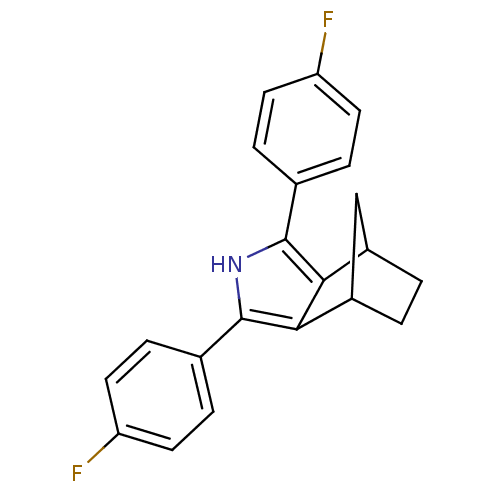
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CCC(C3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C21H17F2N/c22-16-7-3-12(4-8-16)20-18-14-1-2-15(11-14)19(18)21(24-20)13-5-9-17(23)10-6-13/h3-10,14-15,24H,1-2,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition against Prostaglandin G/H synthase 2 in human blood (95% confidence limits) |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272126
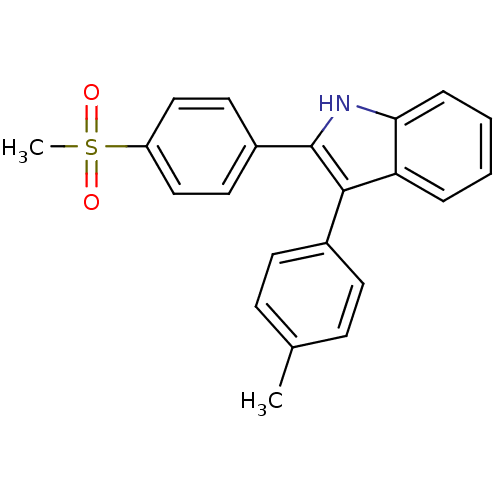
(2-(4-(methylsulfonyl)phenyl)-3-p-tolyl-1H-indole |...)Show SMILES Cc1ccc(cc1)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO2S/c1-15-7-9-16(10-8-15)21-19-5-3-4-6-20(19)23-22(21)17-11-13-18(14-12-17)26(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272105
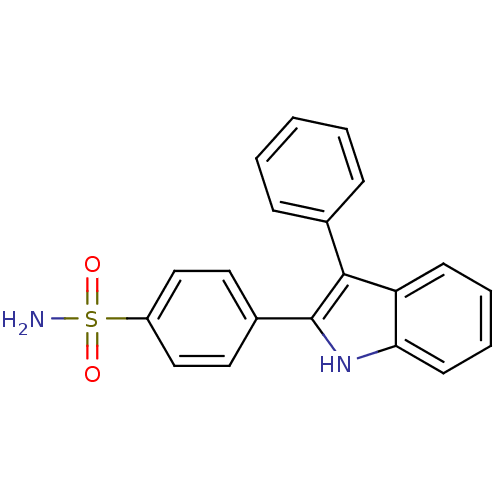
(4-(3-phenyl-1H-indol-2-yl)benzenesulfonamide | CHE...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C20H16N2O2S/c21-25(23,24)16-12-10-15(11-13-16)20-19(14-6-2-1-3-7-14)17-8-4-5-9-18(17)22-20/h1-13,22H,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50094551
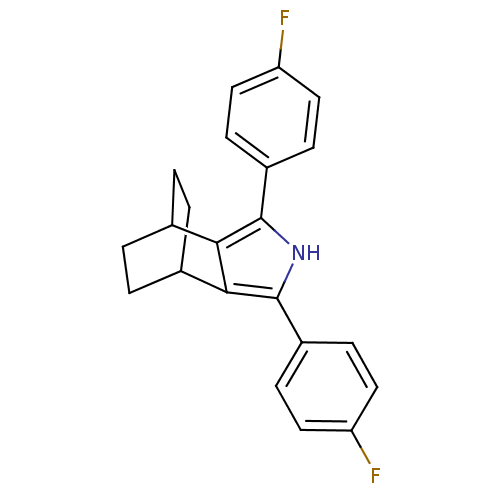
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.2.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CCC(CC3)c12)-c1ccc(F)cc1 |(21.01,-36.41,;22.48,-35.94,;22.8,-34.43,;24.27,-33.96,;25.41,-35,;25.09,-36.5,;23.63,-36.98,;26.88,-34.53,;28.12,-35.45,;29.38,-34.55,;28.9,-33.08,;29.67,-31.77,;28.93,-30.43,;27.39,-30.42,;26.61,-31.74,;27.93,-30.96,;28.33,-32.53,;27.36,-33.07,;30.84,-35.03,;31.15,-36.54,;32.61,-37.02,;33.76,-36,;35.23,-36.48,;33.44,-34.48,;31.98,-34,)| Show InChI InChI=1S/C22H19F2N/c23-17-9-5-15(6-10-17)21-19-13-1-2-14(4-3-13)20(19)22(25-21)16-7-11-18(24)12-8-16/h5-14,25H,1-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition against Prostaglandin G/H synthase 2 in human blood (95% confidence limits) |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272110
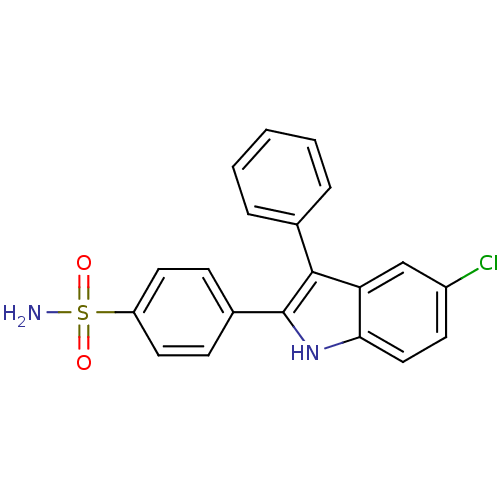
(4-(5-chloro-3-phenyl-1H-indol-2-yl)benzenesulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccccc1 Show InChI InChI=1S/C20H15ClN2O2S/c21-15-8-11-18-17(12-15)19(13-4-2-1-3-5-13)20(23-18)14-6-9-16(10-7-14)26(22,24)25/h1-12,23H,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272097
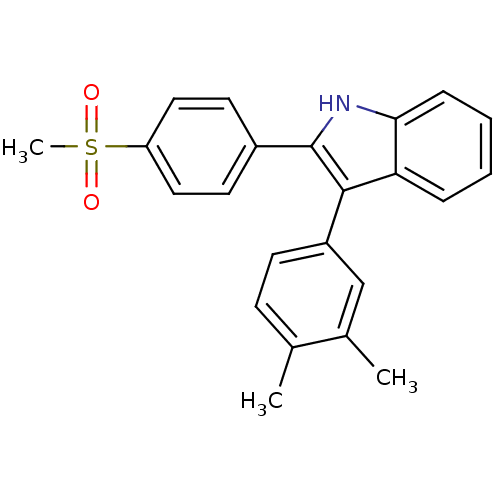
(3-(3,4-dimethylphenyl)-2-(4-(methylsulfonyl)phenyl...)Show SMILES Cc1ccc(cc1C)-c1c([nH]c2ccccc12)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C23H21NO2S/c1-15-8-9-18(14-16(15)2)22-20-6-4-5-7-21(20)24-23(22)17-10-12-19(13-11-17)27(3,25)26/h4-14,24H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272128
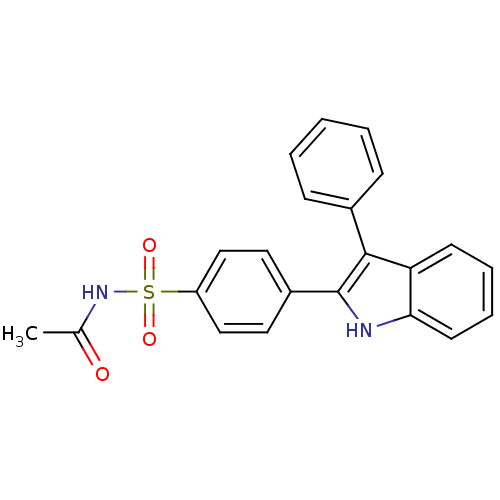
(CHEMBL500943 | N-(4-(3-phenyl-1H-indol-2-yl)phenyl...)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C22H18N2O3S/c1-15(25)24-28(26,27)18-13-11-17(12-14-18)22-21(16-7-3-2-4-8-16)19-9-5-6-10-20(19)23-22/h2-14,23H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM315131
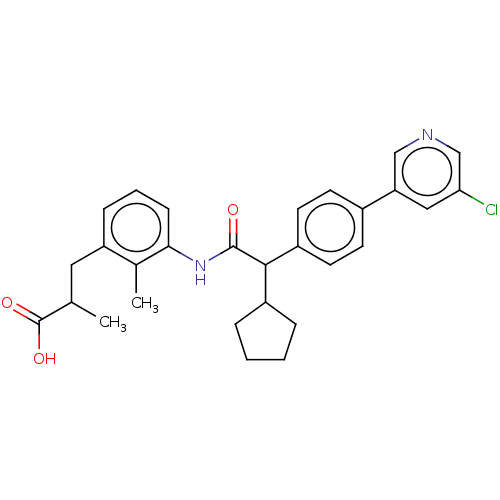
((2R/S)-3-[3-({(2R/S)-2-[4-(5-Chloropyridin-3-yl)ph...)Show SMILES CC(Cc1cccc(NC(=O)C(C2CCCC2)c2ccc(cc2)-c2cncc(Cl)c2)c1C)C(O)=O Show InChI InChI=1S/C29H31ClN2O3/c1-18(29(34)35)14-23-8-5-9-26(19(23)2)32-28(33)27(21-6-3-4-7-21)22-12-10-20(11-13-22)24-15-25(30)17-31-16-24/h5,8-13,15-18,21,27H,3-4,6-7,14H2,1-2H3,(H,32,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... |
US Patent US10172814 (2019)
BindingDB Entry DOI: 10.7270/Q20K2BNW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50365267
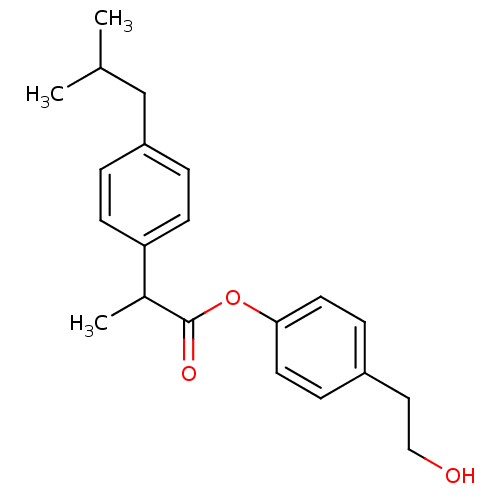
(CHEMBL1958349)Show InChI InChI=1S/C21H26O3/c1-15(2)14-18-4-8-19(9-5-18)16(3)21(23)24-20-10-6-17(7-11-20)12-13-22/h4-11,15-16,22H,12-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 55: 688-96 (2012)
Article DOI: 10.1021/jm200973j
BindingDB Entry DOI: 10.7270/Q2HM58Z7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297677
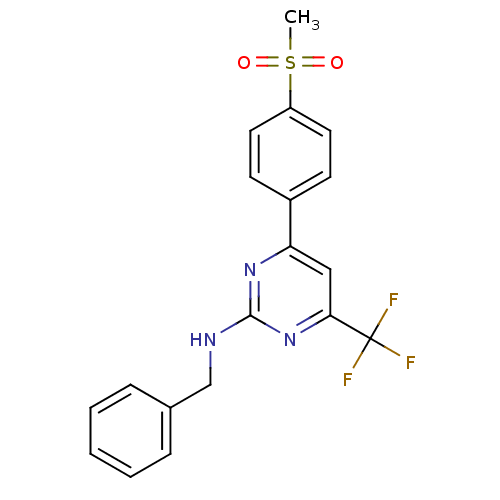
(CHEMBL551829 | N-benzyl-4-(4-(methylsulfonyl)pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O2S/c1-28(26,27)15-9-7-14(8-10-15)16-11-17(19(20,21)22)25-18(24-16)23-12-13-5-3-2-4-6-13/h2-11H,12H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50297675
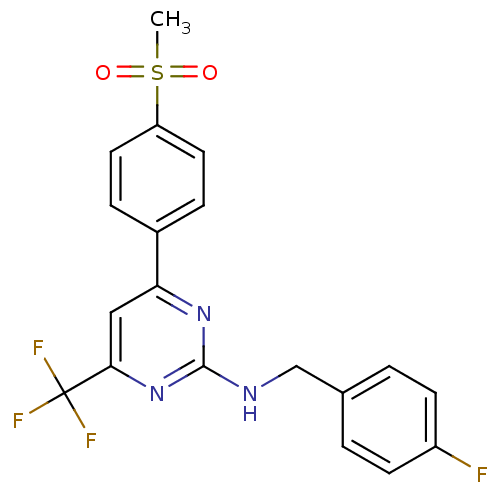
(CHEMBL551148 | N-(4-fluorobenzyl)-4-(4-(methylsulf...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NCc2ccc(F)cc2)n1)C(F)(F)F Show InChI InChI=1S/C19H15F4N3O2S/c1-29(27,28)15-8-4-13(5-9-15)16-10-17(19(21,22)23)26-18(25-16)24-11-12-2-6-14(20)7-3-12/h2-10H,11H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272130
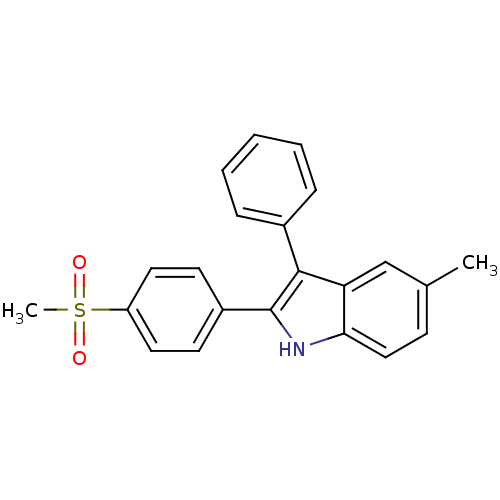
(5-methyl-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...)Show SMILES Cc1ccc2[nH]c(c(-c3ccccc3)c2c1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C22H19NO2S/c1-15-8-13-20-19(14-15)21(16-6-4-3-5-7-16)22(23-20)17-9-11-18(12-10-17)26(2,24)25/h3-14,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50373567

(CHEMBL271173)Show SMILES CCOc1cc(NCc2cccs2)nc(n1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H19N3O3S2/c1-3-24-17-11-16(19-12-14-5-4-10-25-14)20-18(21-17)13-6-8-15(9-7-13)26(2,22)23/h4-11H,3,12H2,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Faes Farma S.A.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 in human whole blood |
Bioorg Med Chem 16: 2183-99 (2008)
Article DOI: 10.1016/j.bmc.2007.11.079
BindingDB Entry DOI: 10.7270/Q2GX4CDQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50100403
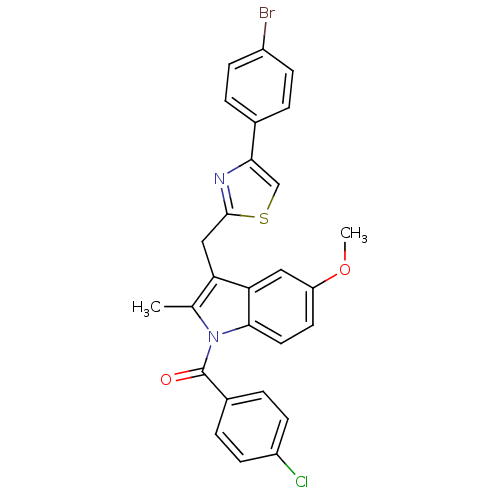
(CHEMBL423296 | {3-[4-(4-Bromo-phenyl)-thiazol-2-yl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(Cc3nc(cs3)-c3ccc(Br)cc3)c2c1 Show InChI InChI=1S/C27H20BrClN2O2S/c1-16-22(14-26-30-24(15-34-26)17-3-7-19(28)8-4-17)23-13-21(33-2)11-12-25(23)31(16)27(32)18-5-9-20(29)10-6-18/h3-13,15H,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) |
Bioorg Med Chem Lett 11: 1325-8 (2001)
BindingDB Entry DOI: 10.7270/Q2PG1R1K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272111
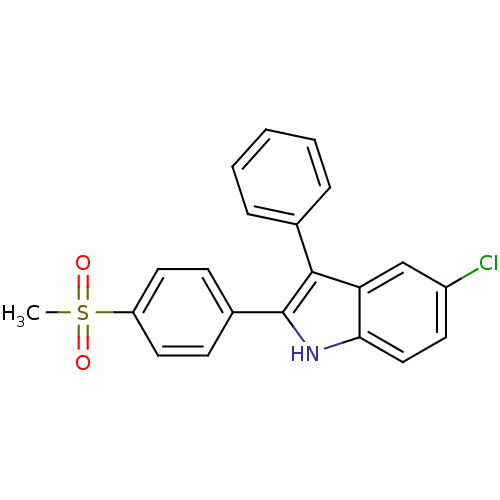
(5-chloro-2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccccc1 Show InChI InChI=1S/C21H16ClNO2S/c1-26(24,25)17-10-7-15(8-11-17)21-20(14-5-3-2-4-6-14)18-13-16(22)9-12-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272124
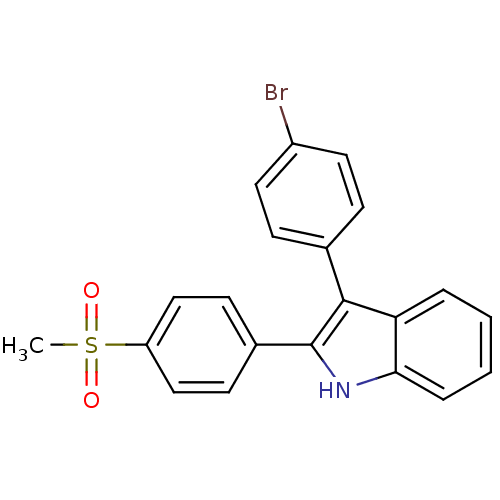
(3-(4-bromophenyl)-2-(4-(methylsulfonyl)phenyl)-1H-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccc(Br)cc1 Show InChI InChI=1S/C21H16BrNO2S/c1-26(24,25)17-12-8-15(9-13-17)21-20(14-6-10-16(22)11-7-14)18-4-2-3-5-19(18)23-21/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50373588

(CHEMBL270293)Show SMILES COc1cc(NCc2cccs2)nc(n1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H17N3O3S2/c1-23-16-10-15(18-11-13-4-3-9-24-13)19-17(20-16)12-5-7-14(8-6-12)25(2,21)22/h3-10H,11H2,1-2H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Faes Farma S.A.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 in human whole blood |
Bioorg Med Chem 16: 2183-99 (2008)
Article DOI: 10.1016/j.bmc.2007.11.079
BindingDB Entry DOI: 10.7270/Q2GX4CDQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50153990
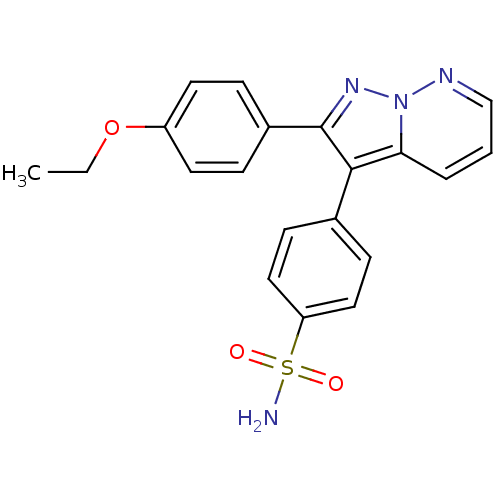
(4-[2-(4-Ethoxy-phenyl)-pyrazolo[1,5-b]pyridazin-3-...)Show SMILES CCOc1ccc(cc1)-c1nn2ncccc2c1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H18N4O3S/c1-2-27-16-9-5-15(6-10-16)20-19(18-4-3-13-22-24(18)23-20)14-7-11-17(12-8-14)28(21,25)26/h3-13H,2H2,1H3,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 expressed in COS cells |
Bioorg Med Chem Lett 14: 5445-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.089
BindingDB Entry DOI: 10.7270/Q2RJ4HZ7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057527
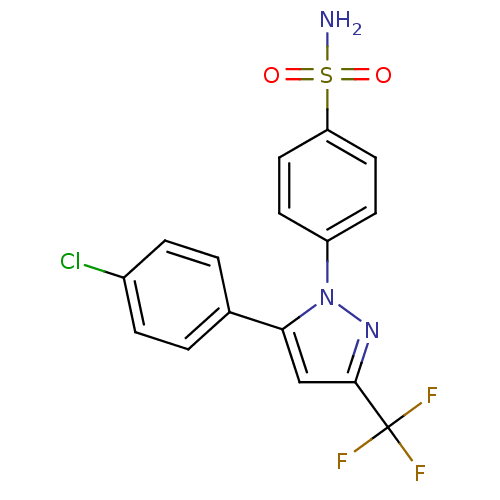
(4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50056998

(4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...)Show SMILES CN1C(C(=O)Nc2ncc(C)s2)=C(O)c2ccccc2S1(=O)=O |t:12| Show InChI InChI=1S/C14H13N3O4S2/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2/h3-7,18H,1-2H3,(H,15,16,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114597
BindingDB Entry DOI: 10.7270/Q2VD73F7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029613
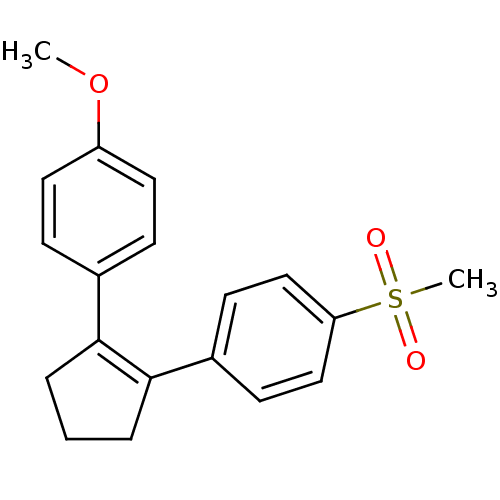
(1-Methoxy-4-(2-(4-(methanesulfonyl)phenyl)cyclopen...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C19H20O3S/c1-22-16-10-6-14(7-11-16)18-4-3-5-19(18)15-8-12-17(13-9-15)23(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 38: 4570-8 (1995)
BindingDB Entry DOI: 10.7270/Q23N22D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
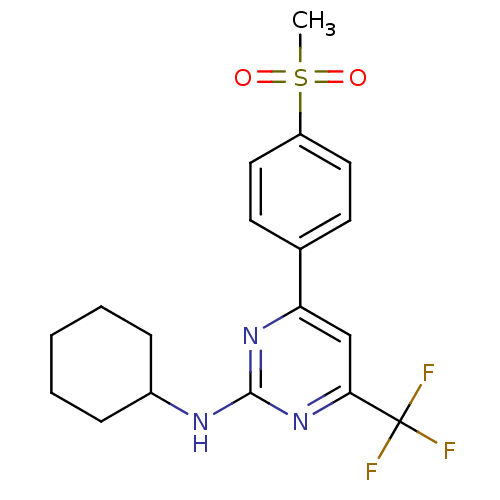
(Homo sapiens (Human)) | BDBM50297669
(CHEMBL561891 | N-cyclohexyl-4-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(nc(NC2CCCCC2)n1)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O2S/c1-27(25,26)14-9-7-12(8-10-14)15-11-16(18(19,20)21)24-17(23-15)22-13-5-3-2-4-6-13/h7-11,13H,2-6H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ... |
Bioorg Med Chem Lett 19: 4504-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.085
BindingDB Entry DOI: 10.7270/Q20Z7396 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272090

(4-(5-chloro-3-(4-chlorophenyl)-1H-indol-2-yl)benze...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H14Cl2N2O2S/c21-14-5-1-12(2-6-14)19-17-11-15(22)7-10-18(17)24-20(19)13-3-8-16(9-4-13)27(23,25)26/h1-11,24H,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
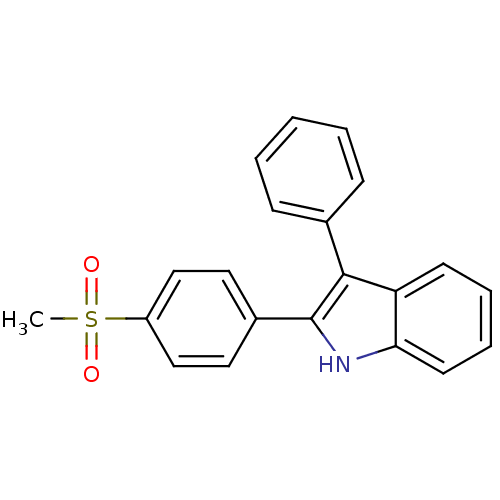
(Homo sapiens (Human)) | BDBM50272106
(2-(4-(methylsulfonyl)phenyl)-3-phenyl-1H-indole | ...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C21H17NO2S/c1-25(23,24)17-13-11-16(12-14-17)21-20(15-7-3-2-4-8-15)18-9-5-6-10-19(18)22-21/h2-14,22H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50610906
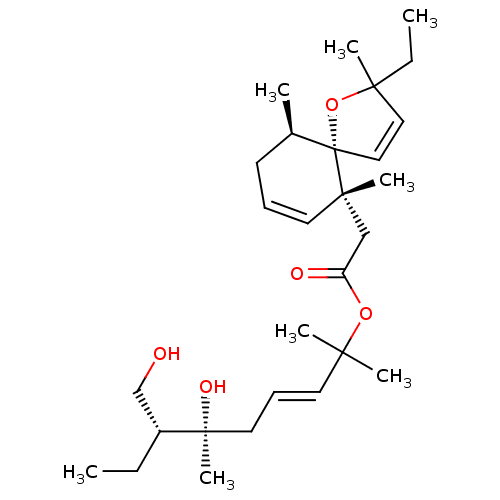
(CHEMBL5267320) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50610905

(CHEMBL5267685) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50610907
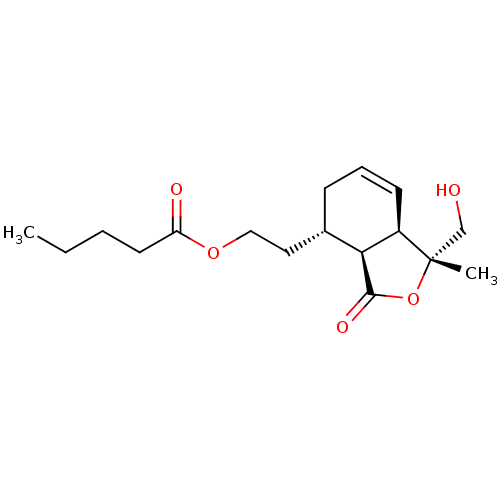
(CHEMBL5270325) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50272092
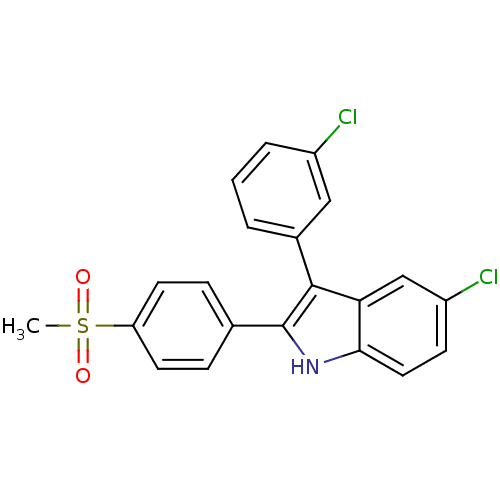
(5-chloro-3-(3-chlorophenyl)-2-(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1[nH]c2ccc(Cl)cc2c1-c1cccc(Cl)c1 Show InChI InChI=1S/C21H15Cl2NO2S/c1-27(25,26)17-8-5-13(6-9-17)21-20(14-3-2-4-15(22)11-14)18-12-16(23)7-10-19(18)24-21/h2-12,24H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology and Science
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 43: 1297-303 (2008)
Article DOI: 10.1016/j.ejmech.2007.06.022
BindingDB Entry DOI: 10.7270/Q2CC10F1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50407503
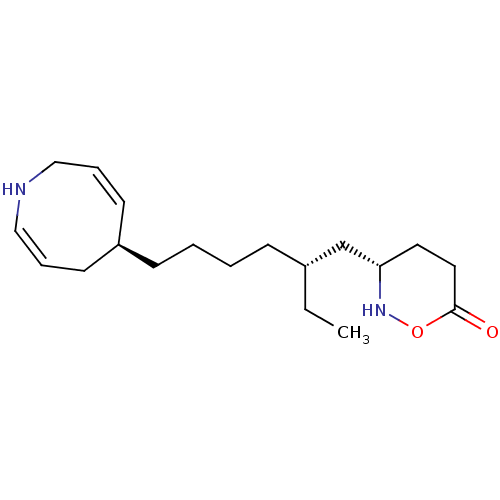
(CHEMBL5274847)Show InChI InChI=1S/C20H24N4S/c1-2-3-4-5-7-14-10-12-15(13-11-14)25-17-9-6-8-16-18(17)19(21)24-20(22)23-16/h6,8-13H,2-5,7H2,1H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity for carbachol induced contractions in guinea pig ileum against Muscarinic acetylcholine receptor M3 in the presence of mepyrami... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049042

(4-[6-(4-Trifluoromethyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C20H18F3NO2S/c21-20(22,23)15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)27(24,25)26/h1-8H,9-12H2,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049011
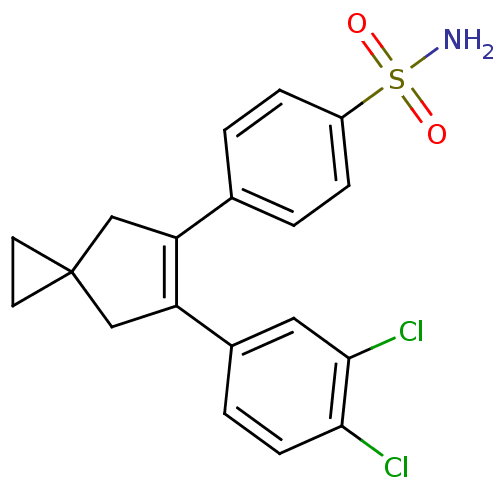
(4-[6-(3,4-Dichloro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17Cl2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50580628
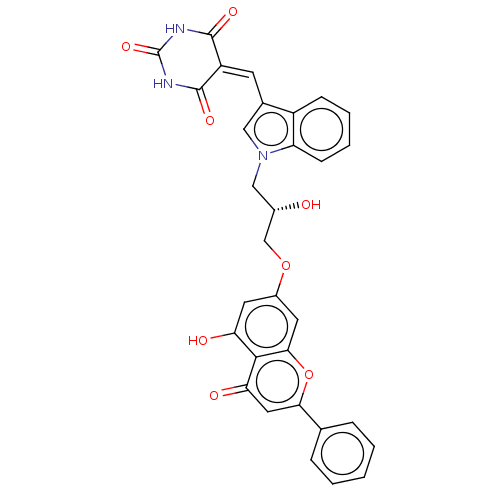
(CHEMBL5089355)Show SMILES [#8]-[#6@H](-[#6]-[#8]-c1cc(-[#8])c2c(c1)oc(cc2=O)-c1ccccc1)-[#6]-n1cc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX-2 using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition and measured after 2 mins by EI... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00880
BindingDB Entry DOI: 10.7270/Q2JH3R2W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049038
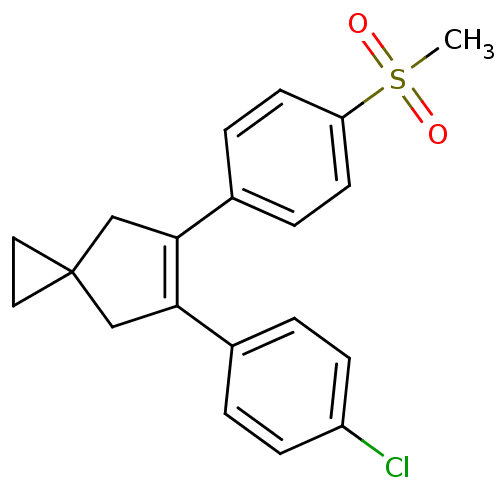
(5-(4-Chloro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C20H19ClO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50601851
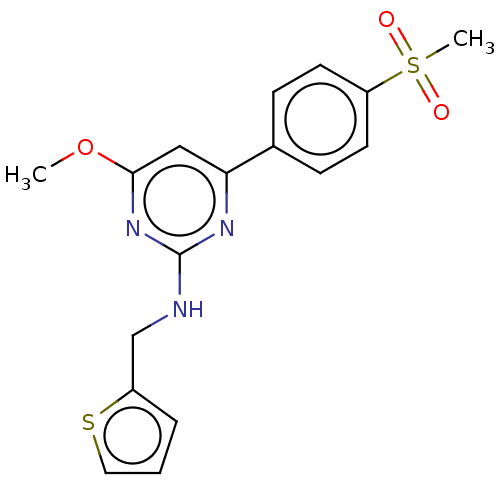
(CHEMBL5200454)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(O[11CH3])nc(NCc2cccs2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50100395
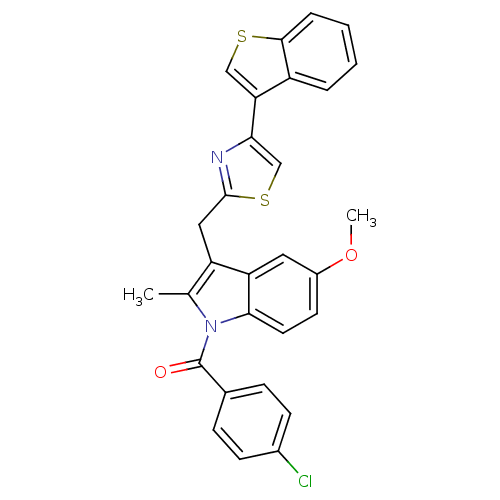
(CHEMBL28155 | [3-(4-Benzo[b]thiophen-3-yl-thiazol-...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(Cc3nc(cs3)-c3csc4ccccc34)c2c1 Show InChI InChI=1S/C29H21ClN2O2S2/c1-17-22(14-28-31-25(16-36-28)24-15-35-27-6-4-3-5-21(24)27)23-13-20(34-2)11-12-26(23)32(17)29(33)18-7-9-19(30)10-8-18/h3-13,15-16H,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) |
Bioorg Med Chem Lett 11: 1325-8 (2001)
BindingDB Entry DOI: 10.7270/Q2PG1R1K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049014

(4-[6-(4-Chloro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C19H18ClNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of human Prostaglandin G/H synthase 2 |
J Med Chem 44: 3223-30 (2001)
BindingDB Entry DOI: 10.7270/Q2736S4D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049025
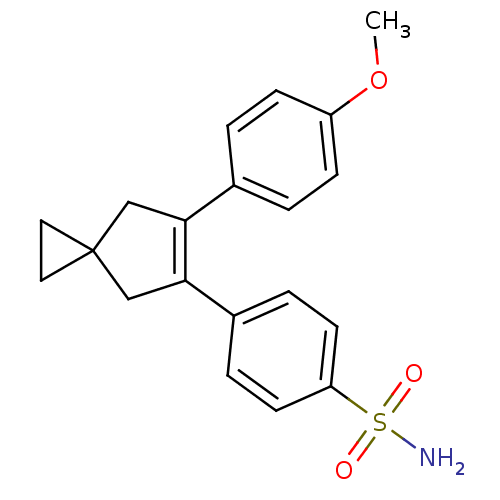
(4-[6-(4-Methoxy-phenyl)-spiro[2.4]hept-5-en-5-yl]-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C20H21NO3S/c1-24-16-6-2-14(3-7-16)18-12-20(10-11-20)13-19(18)15-4-8-17(9-5-15)25(21,22)23/h2-9H,10-13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049025

(4-[6-(4-Methoxy-phenyl)-spiro[2.4]hept-5-en-5-yl]-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C20H21NO3S/c1-24-16-6-2-14(3-7-16)18-12-20(10-11-20)13-19(18)15-4-8-17(9-5-15)25(21,22)23/h2-9H,10-13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of human Prostaglandin G/H synthase 2 |
J Med Chem 44: 3223-30 (2001)
BindingDB Entry DOI: 10.7270/Q2736S4D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049038

(5-(4-Chloro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C20H19ClO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of human Prostaglandin G/H synthase 2 |
J Med Chem 44: 3223-30 (2001)
BindingDB Entry DOI: 10.7270/Q2736S4D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049011

(4-[6-(3,4-Dichloro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17Cl2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2
Curated by ChEMBL
| Assay Description
Inhibition of human Prostaglandin G/H synthase 2 |
J Med Chem 44: 3223-30 (2001)
BindingDB Entry DOI: 10.7270/Q2736S4D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049014

(4-[6-(4-Chloro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C19H18ClNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera
Curated by ChEMBL
| Assay Description
The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells |
Bioorg Med Chem Lett 12: 779-82 (2002)
BindingDB Entry DOI: 10.7270/Q20864MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera
Curated by ChEMBL
| Assay Description
The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 12: 779-82 (2002)
BindingDB Entry DOI: 10.7270/Q20864MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data