

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114154 BindingDB Entry DOI: 10.7270/Q2542SKF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101853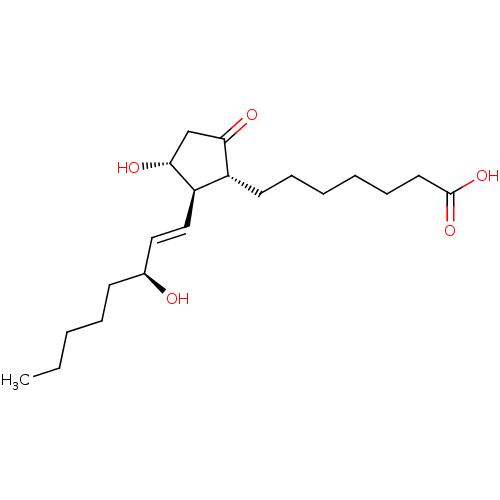 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in human Prostanoid IP receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50594971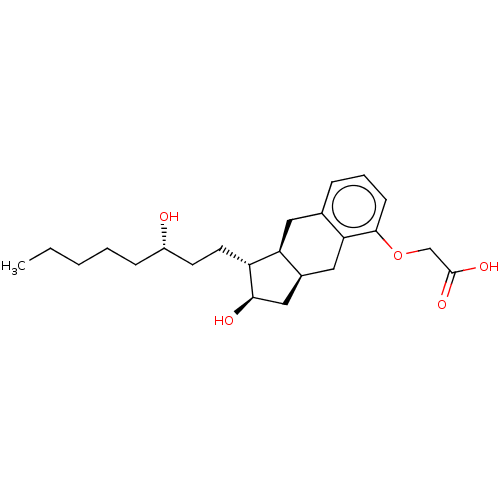 (15-AU-81 | 15AU81 | CHEBI:50861 | L-606 | LRX -15 ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114154 BindingDB Entry DOI: 10.7270/Q2542SKF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357272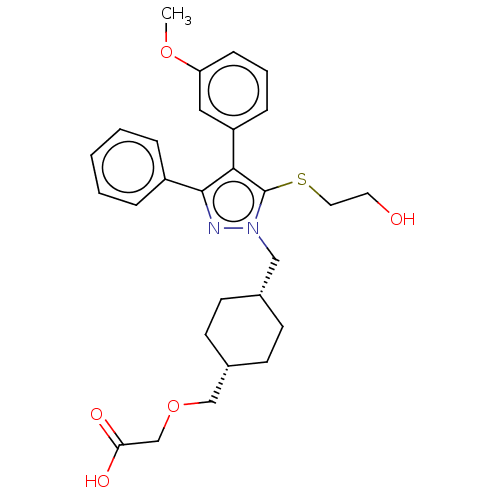 (2-(((1s,4s)-4-((5-(2- hydroxyethylthio)-4-(3- meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA | Assay Description Principle of the assay: The HTRF® assay kit was purchased from Cisbio-US, Inc. (Bedford, Mass.; Catalog #62AM4PEC). The HTRF® assay supported by the ... | Citation and Details BindingDB Entry DOI: 10.7270/Q23N26JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357272 (2-(((1s,4s)-4-((5-(2- hydroxyethylthio)-4-(3- meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description The HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volume... | US Patent US10214518 (2019) BindingDB Entry DOI: 10.7270/Q2T155XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235368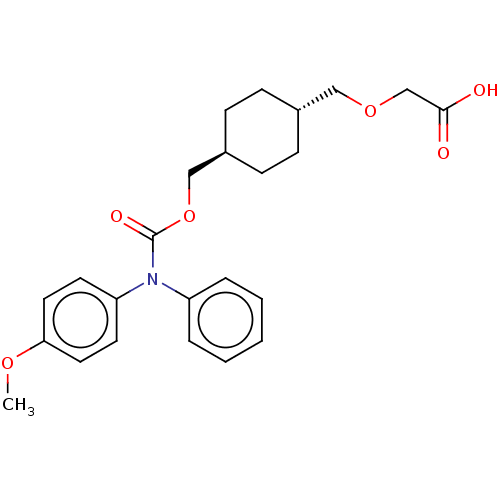 (CHEMBL3893346) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235376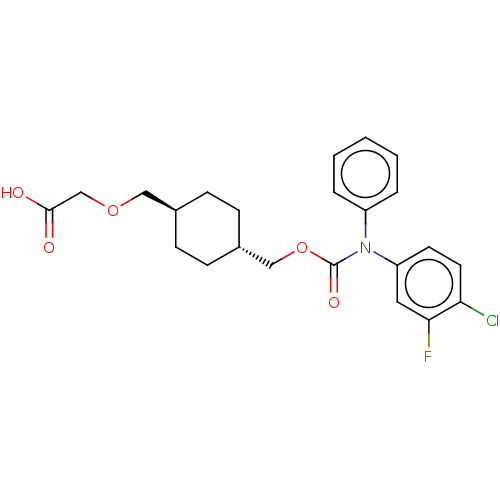 (CHEMBL3926078) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235374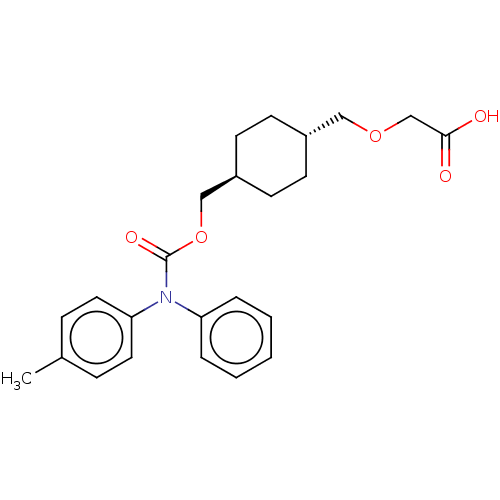 (CHEMBL3935924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103391 (CHEMBL3398233) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235379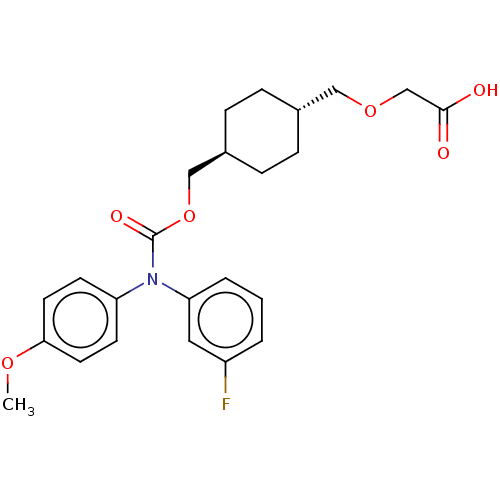 (CHEMBL3932106 | US10668033, Compound 55) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 5.14 | n/a | n/a | n/a | n/a |
ARENA PHARMACEUTICALS, INC. US Patent | Assay Description Compounds were screened for agonists of the human prostacyclin (PGI2) receptor using the HTRF assay for direct cAMP measurement (Gabriel et al., ASSA... | US Patent US10668033 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1PX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235379 (CHEMBL3932106 | US10668033, Compound 55) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235383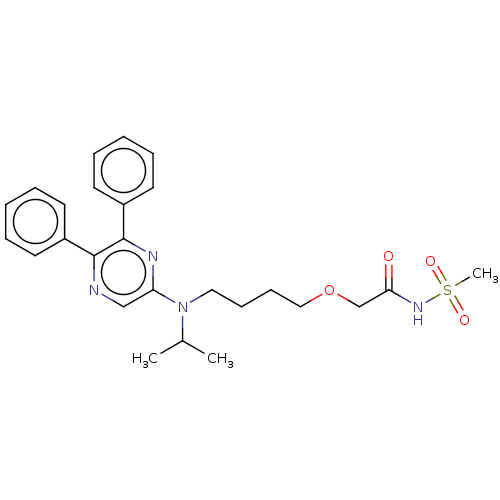 (ACT-293987 | NS-304 | Selexipag | Uptravi) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103396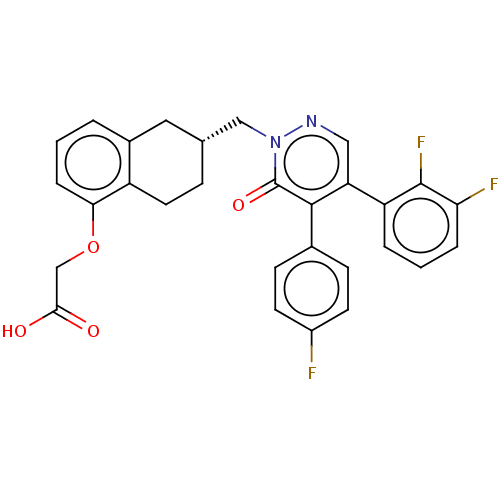 (CHEMBL3398236) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235375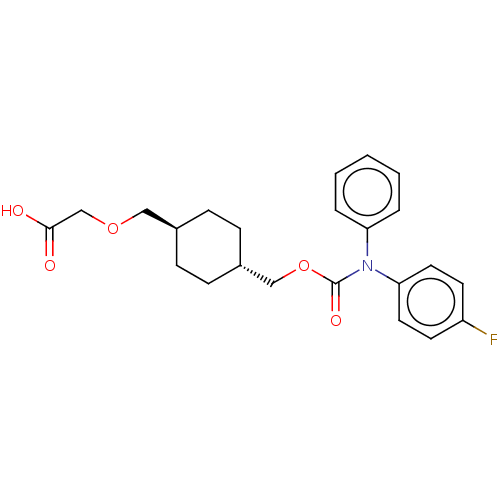 (CHEMBL3975122) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357269 (2-(((1s,4s)-4-((5- (ethylthio)-4-(2-fluoro-3- meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
TBA | Assay Description Principle of the assay: The HTRF® assay kit was purchased from Cisbio-US, Inc. (Bedford, Mass.; Catalog #62AM4PEC). The HTRF® assay supported by the ... | Citation and Details BindingDB Entry DOI: 10.7270/Q23N26JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357269 (2-(((1s,4s)-4-((5- (ethylthio)-4-(2-fluoro-3- meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description The HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volume... | US Patent US10214518 (2019) BindingDB Entry DOI: 10.7270/Q2T155XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235389 (CHEMBL3983767) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Affinity to delta opioid receptor determined in the presence of [3H]- [D-Pen2,5]enkephalin using membranes prepared from rat cerebrum | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM464509 (2-(((1s,4s)-4-((6-(4- methoxyphenyl)-3-oxo-5- phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
ARENA PHARMACEUTICALS, INC. US Patent | Assay Description The HTRF® assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volum... | US Patent US10793529 (2020) BindingDB Entry DOI: 10.7270/Q2K93BMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103405 (CHEMBL3398215) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235370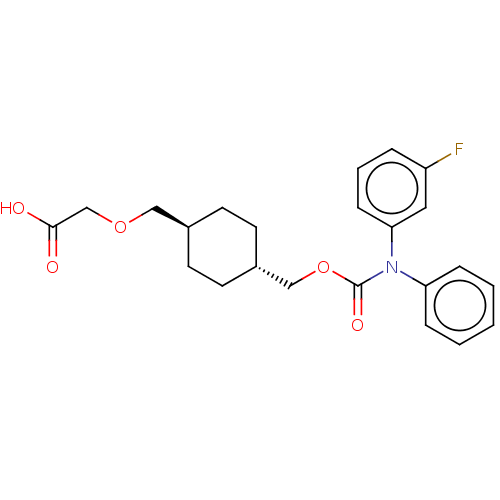 (CHEMBL3933704) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103408 (CHEMBL3398220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103394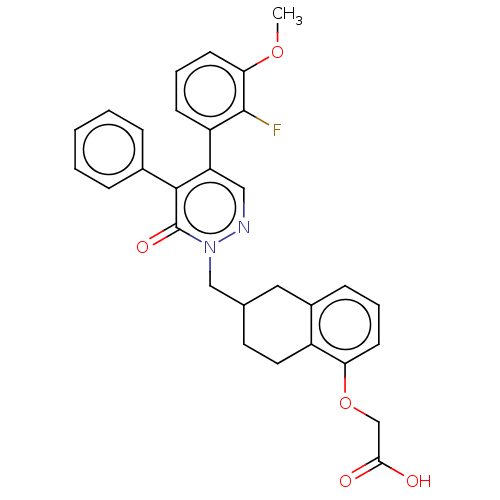 (CHEMBL3398229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235378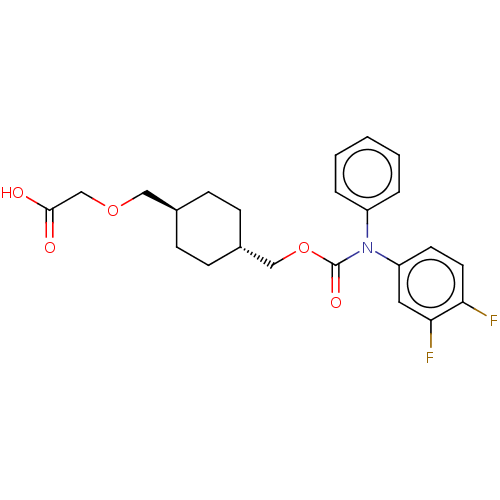 (CHEMBL3981509) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103407 (CHEMBL3398219) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50169552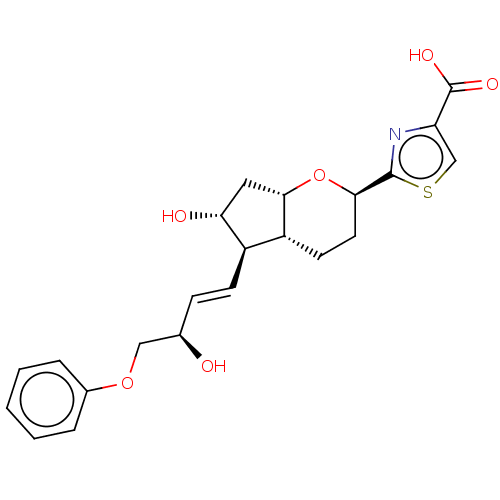 (CHEMBL3804978) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human IP receptor expressed in CHO cells assessed as increase in intracellular cAMP level after 30 mins by HTRF method | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357271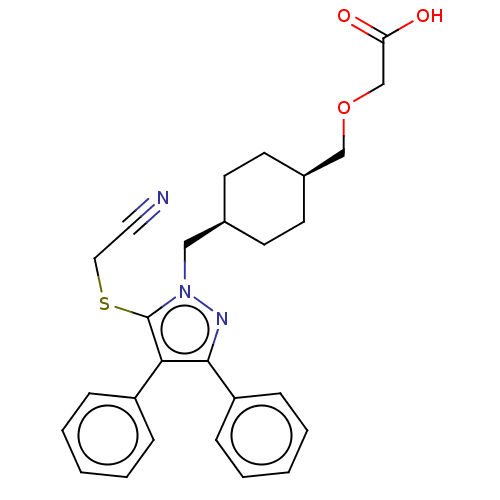 (2-(((1s,4s)-4-((5- (cyanomethylthio)-3,4- diphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description The HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volume... | US Patent US10214518 (2019) BindingDB Entry DOI: 10.7270/Q2T155XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357271 (2-(((1s,4s)-4-((5- (cyanomethylthio)-3,4- diphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA | Assay Description Principle of the assay: The HTRF® assay kit was purchased from Cisbio-US, Inc. (Bedford, Mass.; Catalog #62AM4PEC). The HTRF® assay supported by the ... | Citation and Details BindingDB Entry DOI: 10.7270/Q23N26JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235373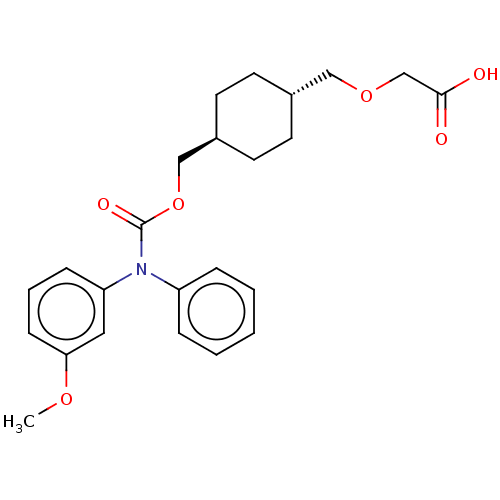 (CHEMBL3928729) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357270 (2-(((1s,4s)-4-((5- (methylthio)-4-(5- methylthioph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA | Assay Description Principle of the assay: The HTRF® assay kit was purchased from Cisbio-US, Inc. (Bedford, Mass.; Catalog #62AM4PEC). The HTRF® assay supported by the ... | Citation and Details BindingDB Entry DOI: 10.7270/Q23N26JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM357270 (2-(((1s,4s)-4-((5- (methylthio)-4-(5- methylthioph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description The HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volume... | US Patent US10214518 (2019) BindingDB Entry DOI: 10.7270/Q2T155XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23963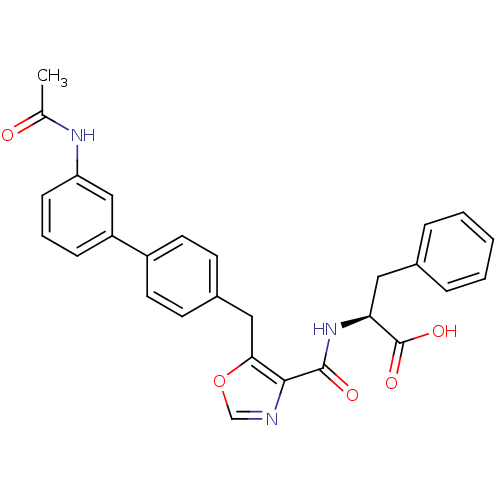 ((2S)-2-[(5-{[4-(3-acetamidophenyl)phenyl]methyl}-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 476 | n/a | 16 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc. | Assay Description IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... | Bioorg Med Chem Lett 17: 1211-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.025 BindingDB Entry DOI: 10.7270/Q2RV0M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235391 (CHEMBL3914174) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235372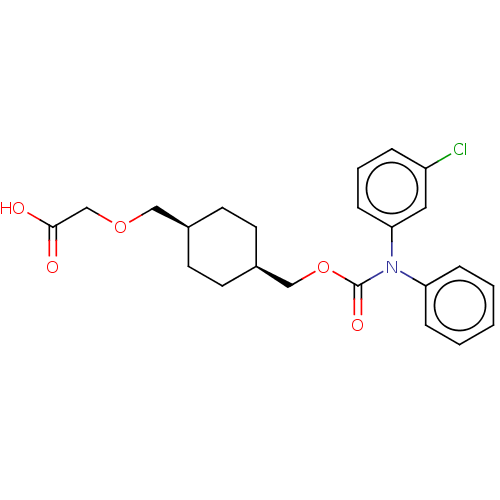 (CHEMBL3966307) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM444835 (2-(((1r,4r)-4-(((3- fluorophenyl)(5-methylthiophen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 19.1 | n/a | n/a | n/a | n/a |
ARENA PHARMACEUTICALS, INC. US Patent | Assay Description Compounds were screened for agonists of the human prostacyclin (PGI2) receptor using the HTRF assay for direct cAMP measurement (Gabriel et al., ASSA... | US Patent US10668033 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1PX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103392 (CHEMBL3398232) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103406 (CHEMBL3398218) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235367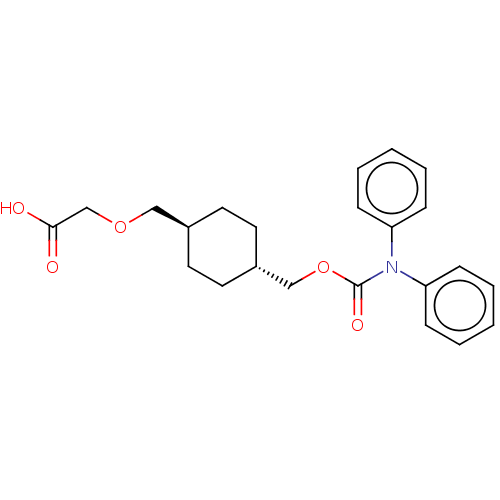 (CHEMBL3952237) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235387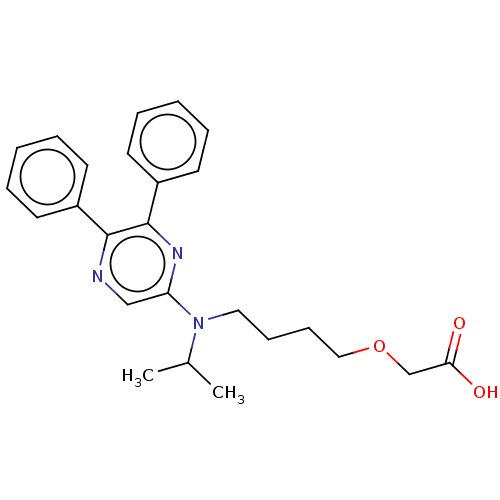 (CHEMBL239226) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235388 (CHEMBL3943791) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103431 (CHEMBL3398216) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101829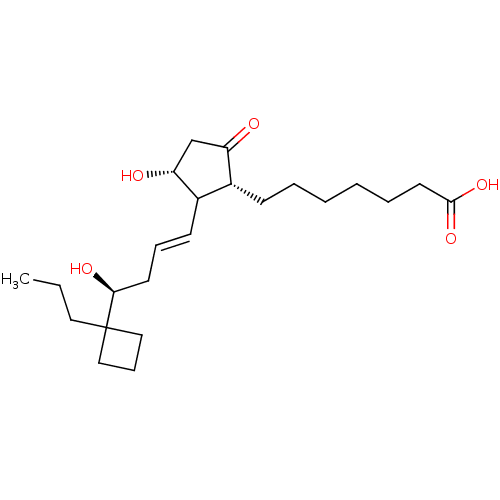 (7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP2 receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235381 (CHEMBL3896580) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50206020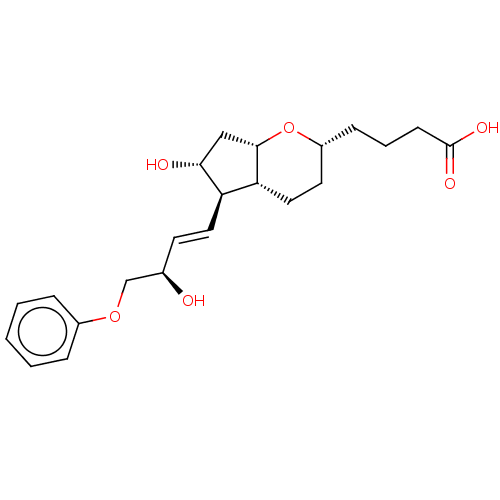 (CHEMBL3982726) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human IP receptor expressed in CHO cells assessed as increase in intracellular cAMP level by HTRF method | ACS Med Chem Lett 8: 107-112 (2017) Article DOI: 10.1021/acsmedchemlett.6b00415 BindingDB Entry DOI: 10.7270/Q2R78H6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM464510 (2-(((1s,4s,)-4-((4-(3-chloro- 2-fluorophenyl)-6-ox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
ARENA PHARMACEUTICALS, INC. US Patent | Assay Description The HTRF® assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volum... | US Patent US10793529 (2020) BindingDB Entry DOI: 10.7270/Q2K93BMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103395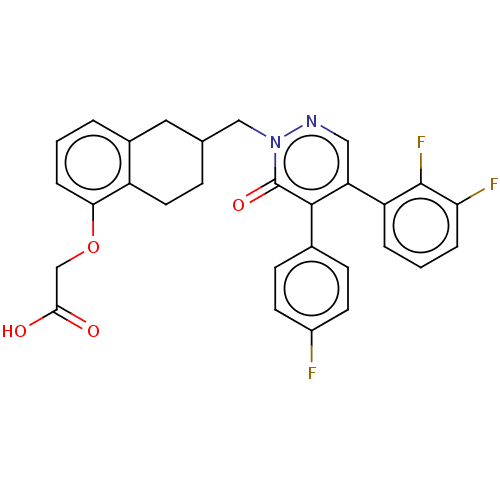 (CHEMBL3398235) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM464511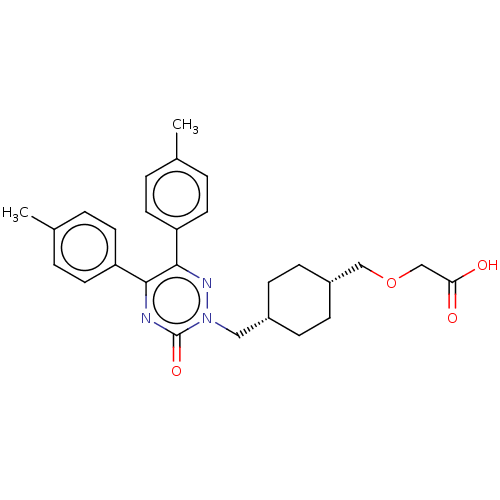 (2-(((1s,4s)-4-((3-oxo-5,6-di- p-tolyl-1,2,4-triazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
ARENA PHARMACEUTICALS, INC. US Patent | Assay Description The HTRF® assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total volum... | US Patent US10793529 (2020) BindingDB Entry DOI: 10.7270/Q2K93BMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101824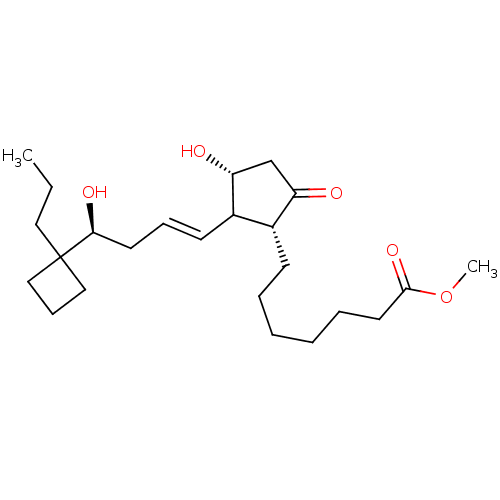 (7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-pro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in human Prostanoid IP receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50103403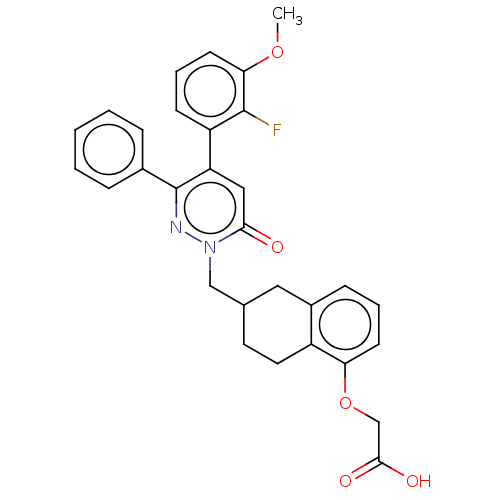 (CHEMBL3398212) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant IP receptor expressed in CHO-K1 cells incubated for 1 hr by HTRF cAMP assay | Bioorg Med Chem Lett 25: 1030-5 (2015) Article DOI: 10.1016/j.bmcl.2015.01.024 BindingDB Entry DOI: 10.7270/Q2057HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 138 total ) | Next | Last >> |