 Found 91 hits of kd for UniProtKB: P30560
Found 91 hits of kd for UniProtKB: P30560 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(RAT) | BDBM35667
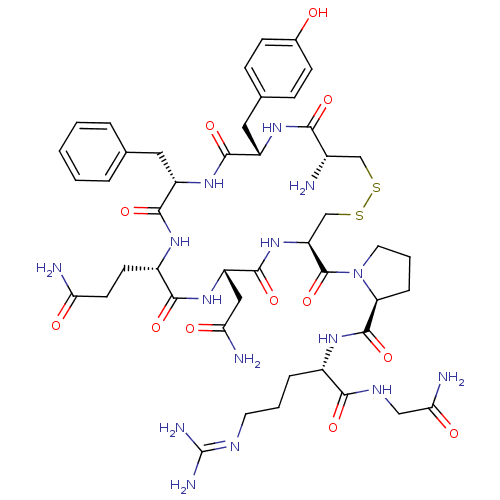
(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against V1 receptor in rat liver cells |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406623

(CHEMBL2115369)Show SMILES COc1ccc(C[C@@H]2NC(=O)C3(CCCC3)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(N)=O)cc1 Show InChI InChI=1S/C58H79N11O12S2/c1-34(2)48-55(78)65-44(31-47(60)71)52(75)66-45(56(79)69-27-11-15-46(69)54(77)62-40(14-7-10-26-59)50(73)63-41(49(61)72)28-36-16-20-38(70)21-17-36)32-82-83-33-58(24-8-9-25-58)57(80)67-43(30-37-18-22-39(81-3)23-19-37)51(74)64-42(53(76)68-48)29-35-12-5-4-6-13-35/h4-6,12-13,16-23,34,40-46,48,70H,7-11,14-15,24-33,59H2,1-3H3,(H2,60,71)(H2,61,72)(H,62,77)(H,63,73)(H,64,74)(H,65,78)(H,66,75)(H,67,80)(H,68,76)/t40-,41-,42-,43-,44-,45-,46-,48-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.182 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008092

(CHEMBL2372415 | cyclo(Ppa-Phe(NO2)-Phe-Val-Asn-Cys...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)C2(CCCC2)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H76N12O13S2/c1-33(2)47-54(78)64-43(30-46(59)71)51(75)65-44(55(79)68-26-10-14-45(68)53(77)61-39(13-6-9-25-58)49(73)62-40(48(60)72)27-36-17-21-38(70)22-18-36)31-83-84-32-57(23-7-8-24-57)56(80)66-42(29-35-15-19-37(20-16-35)69(81)82)50(74)63-41(52(76)67-47)28-34-11-4-3-5-12-34/h3-5,11-12,15-22,33,39-45,47,70H,6-10,13-14,23-32,58H2,1-2H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50406622
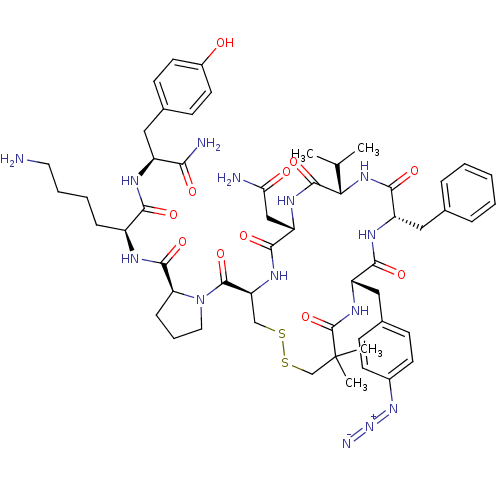
(CHEMBL2115364)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C(C)(C)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C55H74N14O11S2/c1-31(2)45-52(78)63-41(28-44(57)71)49(75)64-42(53(79)69-24-10-14-43(69)51(77)60-37(13-8-9-23-56)47(73)61-38(46(58)72)25-34-17-21-36(70)22-18-34)29-81-82-30-55(3,4)54(80)65-40(27-33-15-19-35(20-16-33)67-68-59)48(74)62-39(50(76)66-45)26-32-11-6-5-7-12-32/h5-7,11-12,15-22,31,37-43,45,70H,8-10,13-14,23-30,56H2,1-4H3,(H2,57,71)(H2,58,72)(H,60,77)(H,61,73)(H,62,74)(H,63,78)(H,64,75)(H,65,80)(H,66,76)/t37-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008096
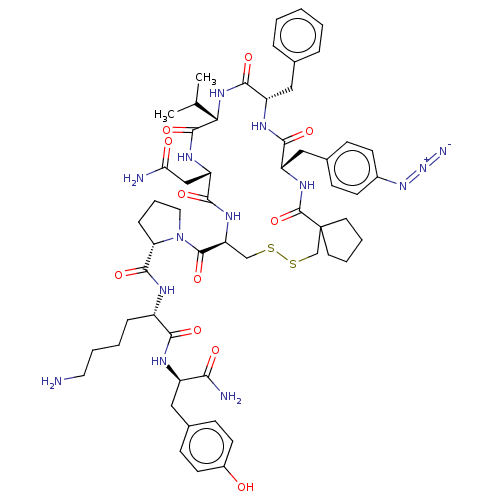
(CHEMBL2372414 | cyclo(Ppa-Phe(N3)-Phe-Val-Asn-Cys)...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)C2(CCCC2)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H76N14O11S2/c1-33(2)47-54(80)65-43(30-46(59)73)51(77)66-44(55(81)71-26-10-14-45(71)53(79)62-39(13-6-9-25-58)49(75)63-40(48(60)74)27-36-17-21-38(72)22-18-36)31-83-84-32-57(23-7-8-24-57)56(82)67-42(29-35-15-19-37(20-16-35)69-70-61)50(76)64-41(52(78)68-47)28-34-11-4-3-5-12-34/h3-5,11-12,15-22,33,39-45,47,72H,6-10,13-14,23-32,58H2,1-2H3,(H2,59,73)(H2,60,74)(H,62,79)(H,63,75)(H,64,76)(H,65,80)(H,66,77)(H,67,82)(H,68,78)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008084
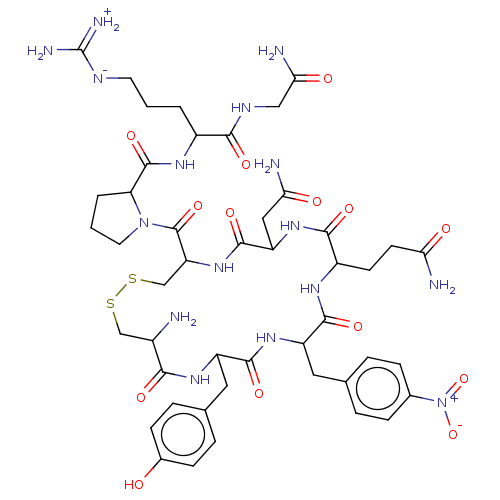
(1-[13-Benzyl-7-carbamoylmethyl-10-isopropyl-16-(4-...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7+](-[#8-])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H64N16O14S2/c47-27-21-77-78-22-33(45(74)61-16-2-4-34(61)44(73)56-28(3-1-15-53-46(51)52)39(68)54-20-37(50)66)60-43(72)32(19-36(49)65)59-40(69)29(13-14-35(48)64)55-41(70)31(17-23-5-9-25(10-6-23)62(75)76)58-42(71)30(57-38(27)67)18-24-7-11-26(63)12-8-24/h5-12,27-34,63H,1-4,13-22,47H2,(H2,48,64)(H2,49,65)(H2,50,66)(H,54,68)(H,55,70)(H,56,73)(H,57,67)(H,58,71)(H,59,69)(H,60,72)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008088

(CHEMBL2372416 | cyclo(Depa-Phe(N3)-Phe-Val-Asn-Cys...)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N14O11S2/c1-5-57(6-2)32-84-83-31-44(55(81)71-26-12-16-45(71)53(79)62-39(15-10-11-25-58)49(75)63-40(48(60)74)27-36-19-23-38(72)24-20-36)66-51(77)43(30-46(59)73)65-54(80)47(33(3)4)68-52(78)41(28-34-13-8-7-9-14-34)64-50(76)42(67-56(57)82)29-35-17-21-37(22-18-35)69-70-61/h7-9,13-14,17-24,33,39-45,47,72H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,73)(H2,60,74)(H,62,79)(H,63,75)(H,64,76)(H,65,80)(H,66,77)(H,67,82)(H,68,78)/t39-,40+,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008083
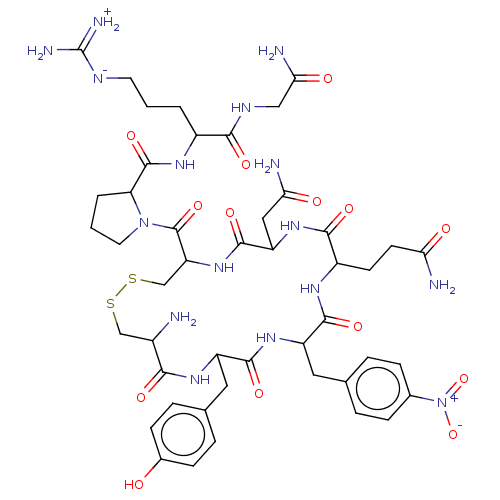
(CHEMBL2372418 | [2-({1-[19-Amino-10-(2-carbamoyl-e...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7+](-[#8-])=O)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N16O14S2/c47-27-21-77-78-22-33(45(74)61-16-2-4-34(61)44(73)56-28(3-1-15-53-46(51)52)39(68)54-20-37(50)66)60-43(72)32(19-36(49)65)59-40(69)29(13-14-35(48)64)55-41(70)31(17-23-5-9-25(10-6-23)62(75)76)58-42(71)30(57-38(27)67)18-24-7-11-26(63)12-8-24/h5-12,27-34,63H,1-4,13-22,47H2,(H2,48,64)(H2,49,65)(H2,50,66)(H,54,68)(H,55,70)(H,56,73)(H,57,67)(H,58,71)(H,59,69)(H,60,72)(H4,51,52,53)/t27-,28-,29-,30+,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008085

(CHEMBL2028984 | [2-({1-[19-Amino-13-(4-azido-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-c2ccncc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O)cc1 |r| Show InChI InChI=1S/C57H82N16O12/c1-5-38(54(82)73-28-12-17-44(73)52(80)68-39(15-10-24-64-56(59)60)48(76)69-40(55(83)84)16-11-25-65-57(61)62)67-50(78)43(32-45(58)74)71-53(81)47(33(3)4)72-51(79)42(29-34-13-8-7-9-14-34)70-49(77)41(30-35-18-20-37(21-19-35)85-6-2)66-46(75)31-36-22-26-63-27-23-36/h7-9,13-14,18-23,26-27,33,38-44,47H,5-6,10-12,15-17,24-25,28-32H2,1-4H3,(H2,58,74)(H,66,75)(H,67,78)(H,68,80)(H,69,76)(H,70,77)(H,71,81)(H,72,79)(H,83,84)(H4,59,60,64)(H4,61,62,65)/t38-,39-,40-,41+,42-,43-,44-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453152

(CHEMBL2115365)Show SMILES CCC1(CC)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC1=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H78N12O13S2/c1-5-57(6-2)32-84-83-31-44(55(79)68-26-12-16-45(68)53(77)61-39(15-10-11-25-58)49(73)62-40(48(60)72)27-36-19-23-38(70)24-20-36)65-51(75)43(30-46(59)71)64-54(78)47(33(3)4)67-52(76)41(28-34-13-8-7-9-14-34)63-50(74)42(66-56(57)80)29-35-17-21-37(22-18-35)69(81)82/h7-9,13-14,17-24,33,39-45,47,70H,5-6,10-12,15-16,25-32,58H2,1-4H3,(H2,59,71)(H2,60,72)(H,61,77)(H,62,73)(H,63,74)(H,64,78)(H,65,75)(H,66,80)(H,67,76)/t39-,40-,41-,42-,43-,44-,45-,47-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Negative log of Kd for Vasopressin V1 receptor |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453150

(CHEMBL2372420)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C53H70N12O13S2/c1-30(2)45-52(75)61-40(28-43(55)67)49(72)62-41(53(76)64-23-8-12-42(64)51(74)58-36(11-6-7-22-54)47(70)59-37(46(56)69)25-33-15-19-35(66)20-16-33)29-80-79-24-21-44(68)57-38(27-32-13-17-34(18-14-32)65(77)78)48(71)60-39(50(73)63-45)26-31-9-4-3-5-10-31/h3-5,9-10,13-20,30,36-42,45,66H,6-8,11-12,21-29,54H2,1-2H3,(H2,55,67)(H2,56,69)(H,57,68)(H,58,74)(H,59,70)(H,60,71)(H,61,75)(H,62,72)(H,63,73)/t36-,37+,38-,39-,40-,41-,42-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453151
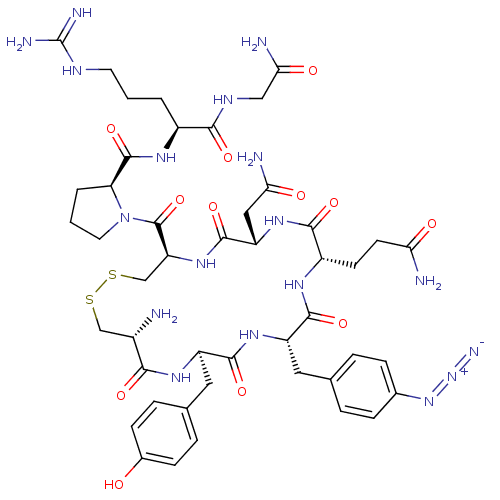
(CHEMBL2372421)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7]=[N+]=[#7-])-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N18O12S2/c47-27-21-77-78-22-33(45(76)64-16-2-4-34(64)44(75)57-28(3-1-15-54-46(51)52)39(70)55-20-37(50)68)61-43(74)32(19-36(49)67)60-40(71)29(13-14-35(48)66)56-41(72)31(17-23-5-9-25(10-6-23)62-63-53)59-42(73)30(58-38(27)69)18-24-7-11-26(65)12-8-24/h5-12,27-34,65H,1-4,13-22,47H2,(H2,48,66)(H2,49,67)(H2,50,68)(H,55,70)(H,56,72)(H,57,75)(H,58,69)(H,59,73)(H,60,71)(H,61,74)(H4,51,52,54)/t27-,28-,29-,30+,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50453148

(CHEMBL2372422)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(cc2)-[#7]=[N+]=[#7-])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H64N18O12S2/c47-27-21-77-78-22-33(45(76)64-16-2-4-34(64)44(75)57-28(3-1-15-54-46(51)52)39(70)55-20-37(50)68)61-43(74)32(19-36(49)67)60-40(71)29(13-14-35(48)66)56-41(72)31(17-23-5-9-25(10-6-23)62-63-53)59-42(73)30(58-38(27)69)18-24-7-11-26(65)12-8-24/h5-12,27-34,65H,1-4,13-22,47H2,(H2,48,66)(H2,49,67)(H2,50,68)(H,55,70)(H,56,72)(H,57,75)(H,58,69)(H,59,73)(H,60,71)(H,61,74)(H4,51,52,54)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038603
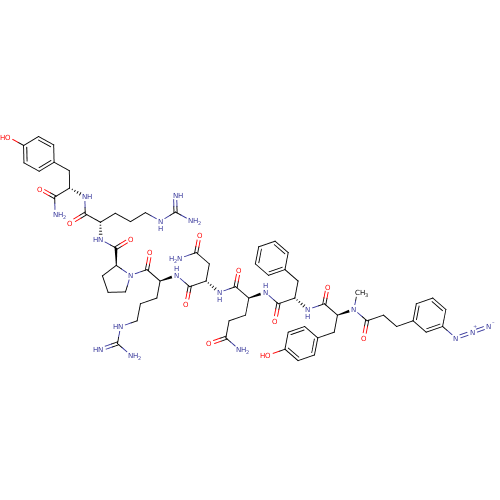
(3-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)27-20-37-11-5-12-40(31-37)80-81-71)50(34-39-18-23-42(85)24-19-39)60(95)79-47(33-36-9-3-2-4-10-36)57(92)74-44(25-26-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(14-7-29-73-63(69)70)61(96)83-30-8-15-49(83)59(94)75-43(13-6-28-72-62(67)68)55(90)77-46(54(66)89)32-38-16-21-41(84)22-17-38/h2-5,9-12,16-19,21-24,31,43-50,84-85H,6-8,13-15,20,25-30,32-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038599
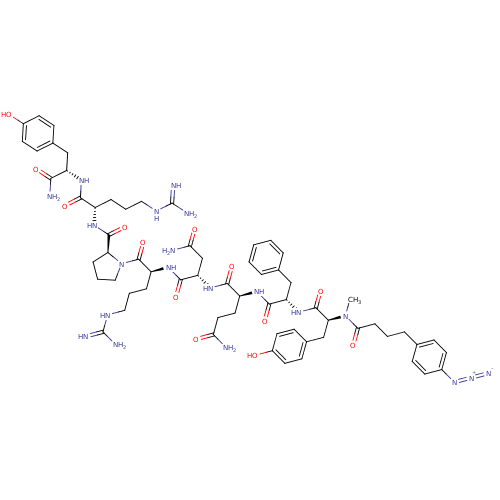
(4-N3-C6H4CH2CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C64H86N20O13/c1-83(54(89)15-5-11-37-16-22-41(23-17-37)81-82-72)51(35-40-20-26-43(86)27-21-40)61(96)80-48(34-38-9-3-2-4-10-38)58(93)75-45(28-29-52(65)87)57(92)79-49(36-53(66)88)59(94)77-46(13-7-31-74-64(70)71)62(97)84-32-8-14-50(84)60(95)76-44(12-6-30-73-63(68)69)56(91)78-47(55(67)90)33-39-18-24-42(85)25-19-39/h2-4,9-10,16-27,44-51,85-86H,5-8,11-15,28-36H2,1H3,(H2,65,87)(H2,66,88)(H2,67,90)(H,75,93)(H,76,95)(H,77,94)(H,78,91)(H,79,92)(H,80,96)(H4,68,69,73)(H4,70,71,74)/t44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038602
(4-N3-C6H4CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-T...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)Cc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C62H82N20O13/c1-81(52(87)33-38-13-19-39(20-14-38)79-80-70)49(32-37-17-23-41(84)24-18-37)59(94)78-46(31-35-8-3-2-4-9-35)56(91)73-43(25-26-50(63)85)55(90)77-47(34-51(64)86)57(92)75-44(11-6-28-72-62(68)69)60(95)82-29-7-12-48(82)58(93)74-42(10-5-27-71-61(66)67)54(89)76-45(53(65)88)30-36-15-21-40(83)22-16-36/h2-4,8-9,13-24,42-49,83-84H,5-7,10-12,25-34H2,1H3,(H2,63,85)(H2,64,86)(H2,65,88)(H,73,91)(H,74,93)(H,75,92)(H,76,89)(H,77,90)(H,78,94)(H4,66,67,71)(H4,68,69,72)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50038601
(4-N3-C6H4CH2CH2CO-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Ar...)Show SMILES CN([C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)CCc1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C63H84N20O13/c1-82(53(88)28-19-36-13-20-40(21-14-36)80-81-71)50(34-39-17-24-42(85)25-18-39)60(95)79-47(33-37-8-3-2-4-9-37)57(92)74-44(26-27-51(64)86)56(91)78-48(35-52(65)87)58(93)76-45(11-6-30-73-63(69)70)61(96)83-31-7-12-49(83)59(94)75-43(10-5-29-72-62(67)68)55(90)77-46(54(66)89)32-38-15-22-41(84)23-16-38/h2-4,8-9,13-18,20-25,43-50,84-85H,5-7,10-12,19,26-35H2,1H3,(H2,64,86)(H2,65,87)(H2,66,89)(H,74,92)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H4,67,68,72)(H4,69,70,73)/t43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS
Curated by ChEMBL
| Assay Description
Tested for dissociation constant at V1a receptor of rat liver membrane |
J Med Chem 37: 1841-9 (1994)
BindingDB Entry DOI: 10.7270/Q2R49PST |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008091
(1-[16-(4-Azido-benzyl)-13-benzyl-7-carbamoylmethyl...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)N=[N+]=[N-])NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C53H70N14O11S2/c1-30(2)45-52(77)62-40(28-43(55)69)49(74)63-41(53(78)67-23-8-12-42(67)51(76)59-36(11-6-7-22-54)47(72)60-37(46(56)71)25-33-15-19-35(68)20-16-33)29-80-79-24-21-44(70)58-38(27-32-13-17-34(18-14-32)65-66-57)48(73)61-39(50(75)64-45)26-31-9-4-3-5-10-31/h3-5,9-10,13-20,30,36-42,45,68H,6-8,11-12,21-29,54H2,1-2H3,(H2,55,69)(H2,56,71)(H,58,70)(H,59,76)(H,60,72)(H,61,73)(H,62,77)(H,63,74)(H,64,75)/t36-,37+,38-,39-,40-,41-,42-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50008097

(CHEMBL2028983 | [2-({1-[19-Amino-13-(4-azido-benzy...)Show SMILES [#6]-[#6@H](-[#7](-[#6])-[#6](=O)-[#6@H]-1-[#6]-[#16]-[#16]-[#6]C2([#6]-[#6]-[#6]-[#6]2)[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-c1ccc(cc1)-[#7]=[N+]=[#7-])-[#6](-[#7])=O |r| Show InChI InChI=1S/C60H83N19O12S2/c1-34(51(84)71-41(14-10-28-69-59(65)66)52(85)70-40(50(64)83)13-6-9-27-68-49(63)37-17-19-38(20-18-37)77-78-67)79(2)57(90)46-32-92-93-33-60(25-7-8-26-60)58(91)76-44(30-36-15-21-39(80)22-16-36)55(88)73-43(29-35-11-4-3-5-12-35)54(87)72-42(23-24-47(61)81)53(86)74-45(31-48(62)82)56(89)75-46/h3-5,11-12,15-22,34,40-46,80H,6-10,13-14,23-33H2,1-2H3,(H2,61,81)(H2,62,82)(H2,63,68)(H2,64,83)(H,70,85)(H,71,84)(H,72,87)(H,73,88)(H,74,86)(H,75,89)(H,76,91)(H4,65,66,69)/t34-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a |
University of Sherbrooke
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 35: 151-7 (1992)
BindingDB Entry DOI: 10.7270/Q2668DT9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016312
(CHEMBL265119 | [Sar7D-Arg8]VP (Vasopressin ))Show SMILES CN(CC(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C44H63N15O12S2/c1-59(20-36(64)53-27(8-5-15-51-44(49)50)38(66)52-19-35(48)63)43(71)32-22-73-72-21-26(45)37(65)55-29(17-24-9-11-25(60)12-10-24)41(69)56-30(16-23-6-3-2-4-7-23)40(68)54-28(13-14-33(46)61)39(67)57-31(18-34(47)62)42(70)58-32/h2-4,6-7,9-12,26-32,60H,5,8,13-22,45H2,1H3,(H2,46,61)(H2,47,62)(H2,48,63)(H,52,66)(H,53,64)(H,54,68)(H,55,65)(H,56,69)(H,57,67)(H,58,70)(H4,49,50,51)/t26-,27-,28-,29-,30+,31+,32+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from bovine kidney inner medulla using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016314
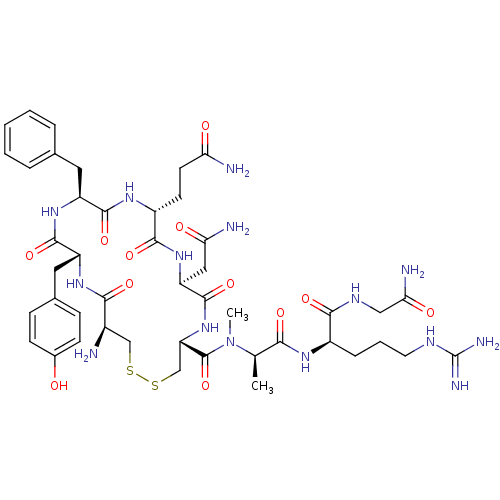
(CHEMBL269715 | [MeAla7D-Arg8]VP (Vasopressin ))Show SMILES C[C@@H](N(C)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H65N15O12S2/c1-23(37(65)54-28(9-6-16-52-45(50)51)39(67)53-20-36(49)64)60(2)44(72)33-22-74-73-21-27(46)38(66)56-30(18-25-10-12-26(61)13-11-25)42(70)57-31(17-24-7-4-3-5-8-24)41(69)55-29(14-15-34(47)62)40(68)58-32(19-35(48)63)43(71)59-33/h3-5,7-8,10-13,23,27-33,61H,6,9,14-22,46H2,1-2H3,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,67)(H,54,65)(H,55,69)(H,56,66)(H,57,70)(H,58,68)(H,59,71)(H4,50,51,52)/t23-,27-,28-,29-,30-,31+,32+,33+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from bovine kidney inner medulla |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016314

(CHEMBL269715 | [MeAla7D-Arg8]VP (Vasopressin ))Show SMILES C[C@@H](N(C)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H65N15O12S2/c1-23(37(65)54-28(9-6-16-52-45(50)51)39(67)53-20-36(49)64)60(2)44(72)33-22-74-73-21-27(46)38(66)56-30(18-25-10-12-26(61)13-11-25)42(70)57-31(17-24-7-4-3-5-8-24)41(69)55-29(14-15-34(47)62)40(68)58-32(19-35(48)63)43(71)59-33/h3-5,7-8,10-13,23,27-33,61H,6,9,14-22,46H2,1-2H3,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,67)(H,54,65)(H,55,69)(H,56,66)(H,57,70)(H,58,68)(H,59,71)(H4,50,51,52)/t23-,27-,28-,29-,30-,31+,32+,33+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from bovine kidney inner medulla |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016312

(CHEMBL265119 | [Sar7D-Arg8]VP (Vasopressin ))Show SMILES CN(CC(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C44H63N15O12S2/c1-59(20-36(64)53-27(8-5-15-51-44(49)50)38(66)52-19-35(48)63)43(71)32-22-73-72-21-26(45)37(65)55-29(17-24-9-11-25(60)12-10-24)41(69)56-30(16-23-6-3-2-4-7-23)40(68)54-28(13-14-33(46)61)39(67)57-31(18-34(47)62)42(70)58-32/h2-4,6-7,9-12,26-32,60H,5,8,13-22,45H2,1H3,(H2,46,61)(H2,47,62)(H2,48,63)(H,52,66)(H,53,64)(H,54,68)(H,55,65)(H,56,69)(H,57,67)(H,58,70)(H4,49,50,51)/t26-,27-,28-,29-,30+,31+,32+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from bovine kidney inner medulla |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016311
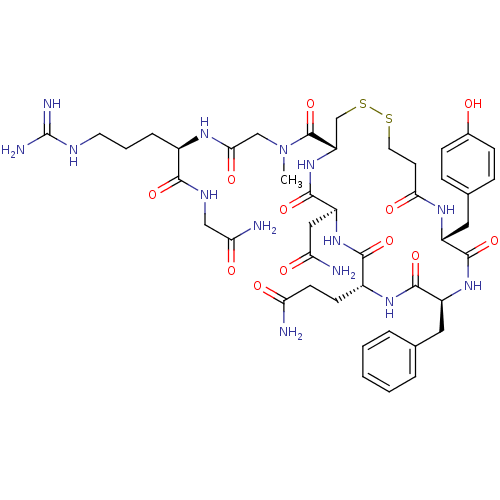
(CHEMBL413249 | [Mpa1Sar7,Drg8]VP (Vasopressin ))Show SMILES CN(CC(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSCCC(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C44H62N14O12S2/c1-58(22-37(64)52-27(8-5-16-50-44(48)49)38(65)51-21-35(47)62)43(70)32-23-72-71-17-15-36(63)53-29(19-25-9-11-26(59)12-10-25)40(67)55-30(18-24-6-3-2-4-7-24)41(68)54-28(13-14-33(45)60)39(66)56-31(20-34(46)61)42(69)57-32/h2-4,6-7,9-12,27-32,59H,5,8,13-23H2,1H3,(H2,45,60)(H2,46,61)(H2,47,62)(H,51,65)(H,52,64)(H,53,63)(H,54,68)(H,55,67)(H,56,66)(H,57,69)(H4,48,49,50)/t27-,28-,29-,30+,31+,32+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from bovine kidney inner medulla using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016315
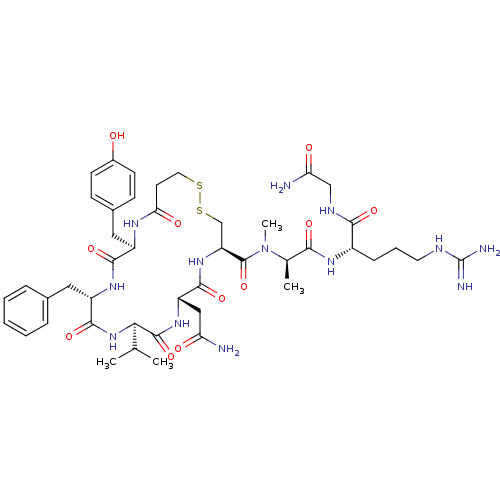
(CHEMBL437631 | [Val4MeAla7]AVP (Arginine-vasopress...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N(C)[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H65N13O11S2/c1-24(2)37-43(68)55-32(21-34(46)60)41(66)56-33(44(69)58(4)25(3)38(63)53-29(11-8-17-50-45(48)49)39(64)51-22-35(47)61)23-71-70-18-16-36(62)52-30(20-27-12-14-28(59)15-13-27)40(65)54-31(42(67)57-37)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,24-25,29-33,37,59H,8,11,16-23H2,1-4H3,(H2,46,60)(H2,47,61)(H,51,64)(H,52,62)(H,53,63)(H,54,65)(H,55,68)(H,56,66)(H,57,67)(H4,48,49,50)/t25-,29+,30-,31+,32+,33+,37-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from bovine kidney inner medulla using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016315

(CHEMBL437631 | [Val4MeAla7]AVP (Arginine-vasopress...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N(C)[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H65N13O11S2/c1-24(2)37-43(68)55-32(21-34(46)60)41(66)56-33(44(69)58(4)25(3)38(63)53-29(11-8-17-50-45(48)49)39(64)51-22-35(47)61)23-71-70-18-16-36(62)52-30(20-27-12-14-28(59)15-13-27)40(65)54-31(42(67)57-37)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,24-25,29-33,37,59H,8,11,16-23H2,1-4H3,(H2,46,60)(H2,47,61)(H,51,64)(H,52,62)(H,53,63)(H,54,65)(H,55,68)(H,56,66)(H,57,67)(H4,48,49,50)/t25-,29+,30-,31+,32+,33+,37-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016317
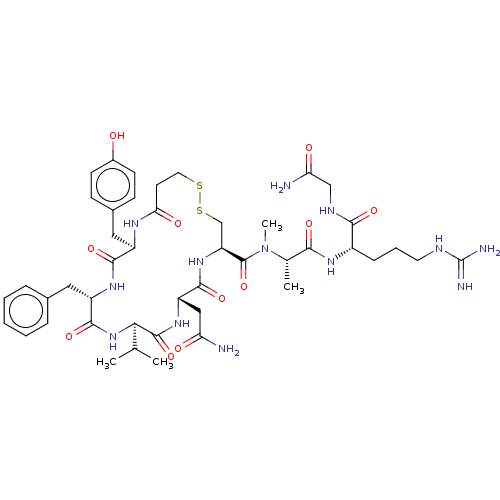
(CHEMBL2371674 | [Mpa1Val4MeAla7]AVP (Arginine-vaso...)Show SMILES [#6]-[#6](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7](-[#6])-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C45H65N13O11S2/c1-24(2)37-43(68)55-32(21-34(46)60)41(66)56-33(44(69)58(4)25(3)38(63)53-29(11-8-17-50-45(48)49)39(64)51-22-35(47)61)23-71-70-18-16-36(62)52-30(20-27-12-14-28(59)15-13-27)40(65)54-31(42(67)57-37)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,24-25,29-33,37,59H,8,11,16-23H2,1-4H3,(H2,46,60)(H2,47,61)(H,51,64)(H,52,62)(H,53,63)(H,54,65)(H,55,68)(H,56,66)(H,57,67)(H4,48,49,50)/t25-,29-,30+,31-,32-,33-,37+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016311

(CHEMBL413249 | [Mpa1Sar7,Drg8]VP (Vasopressin ))Show SMILES CN(CC(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSCCC(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C44H62N14O12S2/c1-58(22-37(64)52-27(8-5-16-50-44(48)49)38(65)51-21-35(47)62)43(70)32-23-72-71-17-15-36(63)53-29(19-25-9-11-26(59)12-10-25)40(67)55-30(18-24-6-3-2-4-7-24)41(68)54-28(13-14-33(45)60)39(66)56-31(20-34(46)61)42(69)57-32/h2-4,6-7,9-12,27-32,59H,5,8,13-23H2,1H3,(H2,45,60)(H2,46,61)(H2,47,62)(H,51,65)(H,52,64)(H,53,63)(H,54,68)(H,55,67)(H,56,66)(H,57,69)(H4,48,49,50)/t27-,28-,29-,30+,31+,32+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016317

(CHEMBL2371674 | [Mpa1Val4MeAla7]AVP (Arginine-vaso...)Show SMILES [#6]-[#6](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7](-[#6])-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C45H65N13O11S2/c1-24(2)37-43(68)55-32(21-34(46)60)41(66)56-33(44(69)58(4)25(3)38(63)53-29(11-8-17-50-45(48)49)39(64)51-22-35(47)61)23-71-70-18-16-36(62)52-30(20-27-12-14-28(59)15-13-27)40(65)54-31(42(67)57-37)19-26-9-6-5-7-10-26/h5-7,9-10,12-15,24-25,29-33,37,59H,8,11,16-23H2,1-4H3,(H2,46,60)(H2,47,61)(H,51,64)(H,52,62)(H,53,63)(H,54,65)(H,55,68)(H,56,66)(H,57,67)(H4,48,49,50)/t25-,29-,30+,31-,32-,33-,37+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016309
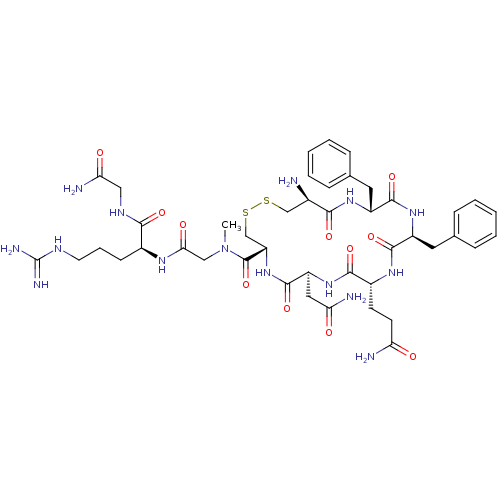
(CHEMBL410025 | [Phe2Sar7]AVP (Arginine-vasopressin...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C44H63N15O11S2/c1-59(21-36(63)53-27(13-8-16-51-44(49)50)38(65)52-20-35(48)62)43(70)32-23-72-71-22-26(45)37(64)55-29(17-24-9-4-2-5-10-24)41(68)56-30(18-25-11-6-3-7-12-25)40(67)54-28(14-15-33(46)60)39(66)57-31(19-34(47)61)42(69)58-32/h2-7,9-12,26-32H,8,13-23,45H2,1H3,(H2,46,60)(H2,47,61)(H2,48,62)(H,52,65)(H,53,63)(H,54,67)(H,55,64)(H,56,68)(H,57,66)(H,58,69)(H4,49,50,51)/t26-,27+,28-,29-,30+,31+,32+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016318
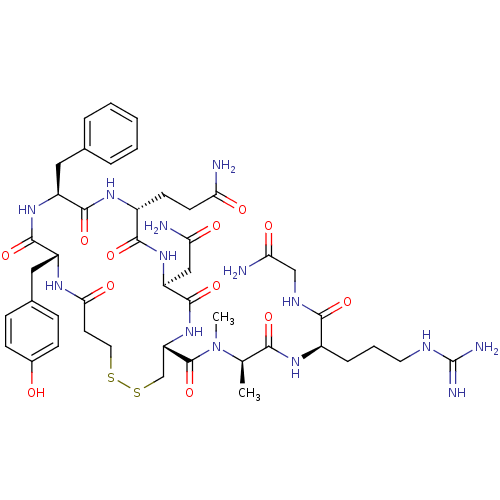
(CHEMBL411357 | [Mpa1,MeAla7,D-Arg8]VP (Vasopressin...)Show SMILES C[C@@H](N(C)C(=O)[C@@H]1CSSCCC(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N14O12S2/c1-24(38(65)54-28(9-6-17-51-45(49)50)39(66)52-22-36(48)63)59(2)44(71)33-23-73-72-18-16-37(64)53-30(20-26-10-12-27(60)13-11-26)41(68)56-31(19-25-7-4-3-5-8-25)42(69)55-29(14-15-34(46)61)40(67)57-32(21-35(47)62)43(70)58-33/h3-5,7-8,10-13,24,28-33,60H,6,9,14-23H2,1-2H3,(H2,46,61)(H2,47,62)(H2,48,63)(H,52,66)(H,53,64)(H,54,65)(H,55,69)(H,56,68)(H,57,67)(H,58,70)(H4,49,50,51)/t24-,28-,29-,30-,31+,32+,33+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016309

(CHEMBL410025 | [Phe2Sar7]AVP (Arginine-vasopressin...)Show SMILES CN(CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1 Show InChI InChI=1S/C44H63N15O11S2/c1-59(21-36(63)53-27(13-8-16-51-44(49)50)38(65)52-20-35(48)62)43(70)32-23-72-71-22-26(45)37(64)55-29(17-24-9-4-2-5-10-24)41(68)56-30(18-25-11-6-3-7-12-25)40(67)54-28(14-15-33(46)60)39(66)57-31(19-34(47)61)42(69)58-32/h2-7,9-12,26-32H,8,13-23,45H2,1H3,(H2,46,60)(H2,47,61)(H2,48,62)(H,52,65)(H,53,63)(H,54,67)(H,55,64)(H,56,68)(H,57,66)(H,58,69)(H4,49,50,51)/t26-,27+,28-,29-,30+,31+,32+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016310
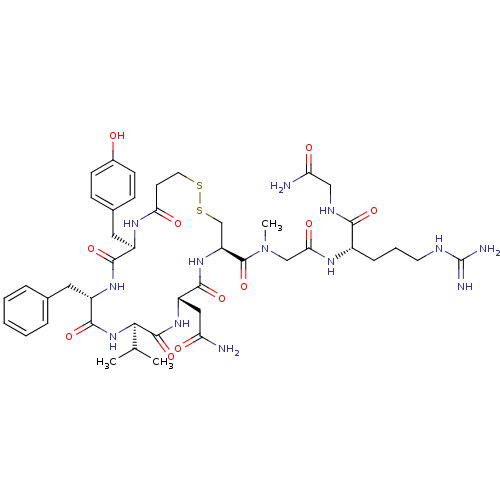
(CHEMBL406303 | [Mpa1Val4Sar7]AVP (Arginine-vasopre...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C44H63N13O11S2/c1-24(2)37-42(67)54-31(20-33(45)59)40(65)55-32(43(68)57(3)22-36(62)51-28(10-7-16-49-44(47)48)38(63)50-21-34(46)60)23-70-69-17-15-35(61)52-29(19-26-11-13-27(58)14-12-26)39(64)53-30(41(66)56-37)18-25-8-5-4-6-9-25/h4-6,8-9,11-14,24,28-32,37,58H,7,10,15-23H2,1-3H3,(H2,45,59)(H2,46,60)(H,50,63)(H,51,62)(H,52,61)(H,53,64)(H,54,67)(H,55,65)(H,56,66)(H4,47,48,49)/t28-,29+,30-,31-,32-,37+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016316
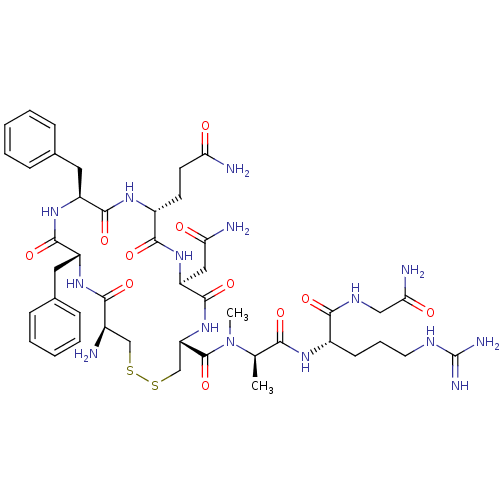
(CHEMBL413706 | [Phe2MeAla7]AVP (Arginine-vasopress...)Show SMILES C[C@@H](N(C)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H65N15O11S2/c1-24(37(64)54-28(14-9-17-52-45(50)51)39(66)53-21-36(49)63)60(2)44(71)33-23-73-72-22-27(46)38(65)56-30(18-25-10-5-3-6-11-25)42(69)57-31(19-26-12-7-4-8-13-26)41(68)55-29(15-16-34(47)61)40(67)58-32(20-35(48)62)43(70)59-33/h3-8,10-13,24,27-33H,9,14-23,46H2,1-2H3,(H2,47,61)(H2,48,62)(H2,49,63)(H,53,66)(H,54,64)(H,55,68)(H,56,65)(H,57,69)(H,58,67)(H,59,70)(H4,50,51,52)/t24-,27-,28+,29-,30-,31+,32+,33+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016316

(CHEMBL413706 | [Phe2MeAla7]AVP (Arginine-vasopress...)Show SMILES C[C@@H](N(C)C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H65N15O11S2/c1-24(37(64)54-28(14-9-17-52-45(50)51)39(66)53-21-36(49)63)60(2)44(71)33-23-73-72-22-27(46)38(65)56-30(18-25-10-5-3-6-11-25)42(69)57-31(19-26-12-7-4-8-13-26)41(68)55-29(15-16-34(47)61)40(67)58-32(20-35(48)62)43(70)59-33/h3-8,10-13,24,27-33H,9,14-23,46H2,1-2H3,(H2,47,61)(H2,48,62)(H2,49,63)(H,53,66)(H,54,64)(H,55,68)(H,56,65)(H,57,69)(H,58,67)(H,59,70)(H4,50,51,52)/t24-,27-,28+,29-,30-,31+,32+,33+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016310

(CHEMBL406303 | [Mpa1Val4Sar7]AVP (Arginine-vasopre...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C44H63N13O11S2/c1-24(2)37-42(67)54-31(20-33(45)59)40(65)55-32(43(68)57(3)22-36(62)51-28(10-7-16-49-44(47)48)38(63)50-21-34(46)60)23-70-69-17-15-35(61)52-29(19-26-11-13-27(58)14-12-26)39(64)53-30(41(66)56-37)18-25-8-5-4-6-9-25/h4-6,8-9,11-14,24,28-32,37,58H,7,10,15-23H2,1-3H3,(H2,45,59)(H2,46,60)(H,50,63)(H,51,62)(H,52,61)(H,53,64)(H,54,67)(H,55,65)(H,56,66)(H4,47,48,49)/t28-,29+,30-,31-,32-,37+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016318

(CHEMBL411357 | [Mpa1,MeAla7,D-Arg8]VP (Vasopressin...)Show SMILES C[C@@H](N(C)C(=O)[C@@H]1CSSCCC(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N14O12S2/c1-24(38(65)54-28(9-6-17-51-45(49)50)39(66)52-22-36(48)63)59(2)44(71)33-23-73-72-18-16-37(64)53-30(20-26-10-12-27(60)13-11-26)41(68)56-31(19-25-7-4-3-5-8-25)42(69)55-29(14-15-34(46)61)40(67)57-32(21-35(47)62)43(70)58-33/h3-5,7-8,10-13,24,28-33,60H,6,9,14-23H2,1-2H3,(H2,46,61)(H2,47,62)(H2,48,63)(H,52,66)(H,53,64)(H,54,65)(H,55,69)(H,56,68)(H,57,67)(H,58,70)(H4,49,50,51)/t24-,28-,29-,30-,31+,32+,33+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016313

(CHEMBL217517 | [Val4Sar7]AVP (Arginine-vasopressin...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C44H64N14O11S2/c1-23(2)36-42(68)55-31(18-33(46)60)40(66)56-32(43(69)58(3)20-35(62)52-28(10-7-15-50-44(48)49)38(64)51-19-34(47)61)22-71-70-21-27(45)37(63)53-29(17-25-11-13-26(59)14-12-25)39(65)54-30(41(67)57-36)16-24-8-5-4-6-9-24/h4-6,8-9,11-14,23,27-32,36,59H,7,10,15-22,45H2,1-3H3,(H2,46,60)(H2,47,61)(H,51,64)(H,52,62)(H,53,63)(H,54,65)(H,55,68)(H,56,66)(H,57,67)(H4,48,49,50)/t27-,28+,29-,30+,31+,32+,36-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50016313

(CHEMBL217517 | [Val4Sar7]AVP (Arginine-vasopressin...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C44H64N14O11S2/c1-23(2)36-42(68)55-31(18-33(46)60)40(66)56-32(43(69)58(3)20-35(62)52-28(10-7-15-50-44(48)49)38(64)51-19-34(47)61)22-71-70-21-27(45)37(63)53-29(17-25-11-13-26(59)14-12-25)39(65)54-30(41(67)57-36)16-24-8-5-4-6-9-24/h4-6,8-9,11-14,23,27-32,36,59H,7,10,15-22,45H2,1-3H3,(H2,46,60)(H2,47,61)(H,51,64)(H,52,62)(H,53,63)(H,54,65)(H,55,68)(H,56,66)(H,57,67)(H4,48,49,50)/t27-,28+,29-,30+,31+,32+,36-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against plasma membrane from rat liver using [3H]-AVP as a radioligand |
J Med Chem 29: 96-9 (1986)
BindingDB Entry DOI: 10.7270/Q2P849V1 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50075817

(CHEMBL2371240 | Mpa-Car-lle-Gln-Asn-Cys-Sar-Arg-Gl...)Show SMILES [H][C@]12N(CCc3c1[nH]c1ccccc31)C(=O)CCSSC[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@]([H])(NC2=O)[C@@H](C)CC)C(=O)N(C)CC(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O |wU:59.62,42.45,1.0,wD:21.54,25.28,33.36,47.51,(5.28,-12.92,;7.12,-12.63,;8.46,-11.86,;8.46,-10.32,;7.12,-9.55,;5.8,-10.32,;5.8,-11.86,;4.32,-12.34,;3.42,-11.09,;1.89,-10.93,;1.27,-9.52,;2.17,-8.27,;3.7,-8.44,;4.32,-9.85,;9.97,-11.53,;9.88,-10,;11.49,-11.7,;12.91,-12.33,;14.05,-13.35,;14.82,-14.69,;15.14,-16.21,;14.98,-17.73,;14.35,-19.14,;13.33,-20.28,;14.28,-21.17,;11.99,-21.06,;12.54,-22.49,;14.06,-22.73,;14.61,-24.17,;15.1,-21.86,;10.49,-21.38,;8.95,-21.21,;8.55,-22.7,;7.54,-20.58,;6.71,-21.87,;7.4,-23.25,;6.57,-24.54,;5.03,-24.46,;7.26,-25.92,;6.4,-19.54,;5.62,-18.22,;4.19,-18.78,;5.31,-16.72,;4.02,-15.37,;5.46,-15.18,;6.1,-13.77,;4.91,-13.32,;3.77,-16.8,;2.93,-15.51,;3.07,-18.16,;1.52,-18.26,;16.47,-18.12,;17.56,-17.04,;16.87,-19.61,;15.77,-20.71,;18.36,-20.01,;19.45,-18.93,;19.05,-17.44,;20.93,-19.33,;22.01,-18.24,;21.62,-16.75,;22.71,-15.66,;22.31,-14.16,;23.4,-13.07,;23.01,-11.6,;24.08,-10.51,;21.51,-11.2,;23.5,-18.64,;23.9,-20.13,;24.6,-17.54,;26.09,-17.94,;27.17,-16.86,;28.67,-17.25,;26.78,-15.37,)| Show InChI InChI=1S/C44H65N15O11S2/c1-4-22(2)35-41(68)54-27(11-12-30(45)60)39(66)55-28(18-31(46)61)40(67)56-29(43(70)58(3)20-33(63)52-26(10-7-15-50-44(48)49)38(65)51-19-32(47)62)21-72-71-17-14-34(64)59-16-13-24-23-8-5-6-9-25(23)53-36(24)37(59)42(69)57-35/h5-6,8-9,22,26-29,35,37,53H,4,7,10-21H2,1-3H3,(H2,45,60)(H2,46,61)(H2,47,62)(H,51,65)(H,52,63)(H,54,68)(H,55,66)(H,56,67)(H,57,69)(H4,48,49,50)/t22-,26-,27-,28-,29+,35-,37-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
Albert Szent-Gy£rgyi Medical University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its dissociation constant (Kd) to rat liver Vasopressin V1 receptor |
Bioorg Med Chem Lett 9: 667-72 (1999)
BindingDB Entry DOI: 10.7270/Q2QC040K |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407335

(CHEMBL428187)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35-,36-,41+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407333
(CHEMBL412942)Show SMILES CCN1[C@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38-,39-,40+,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407338
(CHEMBL385062)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C51H73N13O10S2/c1-30(2)42-48(73)61-36(26-39(52)65)45(70)62-37(49(74)64-23-13-19-38(64)47(72)59-33(18-12-22-56-50(54)55)43(68)57-28-40(53)66)29-75-76-51(20-10-5-11-21-51)27-41(67)58-34(24-31-14-6-3-7-15-31)44(69)60-35(46(71)63-42)25-32-16-8-4-9-17-32/h3-4,6-9,14-17,30,33-38,42H,5,10-13,18-29H2,1-2H3,(H2,52,65)(H2,53,66)(H,57,68)(H,58,67)(H,59,72)(H,60,69)(H,61,73)(H,62,70)(H,63,71)(H4,54,55,56)/t33-,34+,35+,36+,37+,38-,42-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407340
(CHEMBL1790310)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C54H79N11O13S2/c1-5-30(2)44-51(76)63-45(31(3)66)52(77)60-38(27-42(56)69)48(73)61-39(29-79-80-54(21-7-6-8-22-54)28-43(70)64(4)41(50(75)62-44)26-33-15-19-35(68)20-16-33)53(78)65-24-10-12-40(65)49(74)58-36(11-9-23-55)47(72)59-37(46(57)71)25-32-13-17-34(67)18-14-32/h13-20,30-31,36-41,44-45,66-68H,5-12,21-29,55H2,1-4H3,(H2,56,69)(H2,57,71)(H,58,74)(H,59,72)(H,60,77)(H,61,73)(H,62,75)(H,63,76)/t30-,31+,36-,37-,38+,39+,40-,41-,44+,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407341

(CHEMBL264449)Show SMILES CCN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C53H77N13O11S2/c1-4-65-40(26-33-17-19-34(67)20-18-33)49(75)61-36(25-32-13-7-5-8-14-32)47(73)64-44(31(2)3)50(76)62-37(27-41(54)68)46(72)63-38(30-78-79-53(28-43(65)70)21-9-6-10-22-53)51(77)66-24-12-16-39(66)48(74)60-35(15-11-23-58-52(56)57)45(71)59-29-42(55)69/h5,7-8,13-14,17-20,31,35-40,44,67H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,68)(H2,55,69)(H,59,71)(H,60,74)(H,61,75)(H,62,76)(H,63,72)(H,64,73)(H4,56,57,58)/t35-,36+,37+,38+,39-,40-,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407342

(CHEMBL415160)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37-,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Compound was evaluated for the anti-vasopressor activity at V1a receptor. . |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407343
(CHEMBL412806)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C50H75N15O11S2/c1-3-27(2)41-47(75)61-32(15-16-37(51)66)43(71)62-34(23-38(52)67)44(72)63-35(48(76)65-20-10-14-36(65)46(74)60-31(13-9-19-56-49(54)55)42(70)57-25-39(53)68)26-77-78-50(17-7-4-8-18-50)24-40(69)59-33(45(73)64-41)22-29-21-28-11-5-6-12-30(28)58-29/h5-6,11-12,21,27,31-36,41,58H,3-4,7-10,13-20,22-26H2,1-2H3,(H2,51,66)(H2,52,67)(H2,53,68)(H,57,70)(H,59,69)(H,60,74)(H,61,75)(H,62,71)(H,63,72)(H,64,73)(H4,54,55,56)/t27-,31-,32-,33-,34+,35+,36-,41+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407336

(CHEMBL412616)Show SMILES CN1[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CC(N)=O)C(=O)N[C@H](CSSC2(CCCCC2)CC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C52H74N14O12S2/c1-65-39(25-31-14-16-32(67)17-15-31)49(77)63-35(24-30-10-4-2-5-11-30)46(74)60-34(18-19-40(53)68)45(73)62-36(26-41(54)69)47(75)64-37(29-79-80-52(27-43(65)71)20-6-3-7-21-52)50(78)66-23-9-13-38(66)48(76)61-33(12-8-22-58-51(56)57)44(72)59-28-42(55)70/h2,4-5,10-11,14-17,33-39,67H,3,6-9,12-13,18-29H2,1H3,(H2,53,68)(H2,54,69)(H2,55,70)(H,59,72)(H,60,74)(H,61,76)(H,62,73)(H,63,77)(H,64,75)(H4,56,57,58)/t33-,34-,35+,36+,37+,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407337
(CHEMBL1790314)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2ccc(O)cc2)N(C)C(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C45H69N9O12S2/c1-5-25(2)36-41(62)52-37(26(3)55)42(63)49-30(22-34(47)57)38(59)50-31(43(64)54-20-10-12-32(54)39(60)48-29(44(65)66)11-9-19-46)24-67-68-45(17-7-6-8-18-45)23-35(58)53(4)33(40(61)51-36)21-27-13-15-28(56)16-14-27/h13-16,25-26,29-33,36-37,55-56H,5-12,17-24,46H2,1-4H3,(H2,47,57)(H,48,60)(H,49,63)(H,50,59)(H,51,61)(H,52,62)(H,65,66)/t25-,26+,29-,30+,31-,32-,33-,36+,37-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50407348
(CHEMBL1790312)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)CC2(CCCCC2)SSC[C@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(O)=O Show InChI InChI=1S/C46H68N10O11S2/c1-4-25(2)37-42(63)55-38(26(3)57)43(64)52-32(22-35(48)58)39(60)53-33(44(65)56-19-11-15-34(56)41(62)51-30(45(66)67)14-10-18-47)24-68-69-46(16-8-5-9-17-46)23-36(59)50-31(40(61)54-37)21-28-20-27-12-6-7-13-29(27)49-28/h6-7,12-13,20,25-26,30-34,37-38,49,57H,4-5,8-11,14-19,21-24,47H2,1-3H3,(H2,48,58)(H,50,59)(H,51,62)(H,52,64)(H,53,60)(H,54,61)(H,55,63)(H,66,67)/t25-,26+,30-,31-,32+,33-,34-,37+,38-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Anti-vasopressor activity at V1a receptor |
J Med Chem 38: 1762-9 (1995)
BindingDB Entry DOI: 10.7270/Q24M95QZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data