 Found 265 hits of ec50 for UniProtKB: P28702
Found 265 hits of ec50 for UniProtKB: P28702 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50490451

(CHEMBL2325917)Show SMILES CN(c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H29NO2/c1-15-13-18-19(23(4,5)12-11-22(18,2)3)14-20(15)24(6)17-9-7-16(8-10-17)21(25)26/h7-10,13-14H,11-12H2,1-6H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Agonist activity at RXR (unknown origin) in presence of RAR agonist Am80 |
Bioorg Med Chem Lett 23: 81-4 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.008
BindingDB Entry DOI: 10.7270/Q20C4ZPG |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50490452
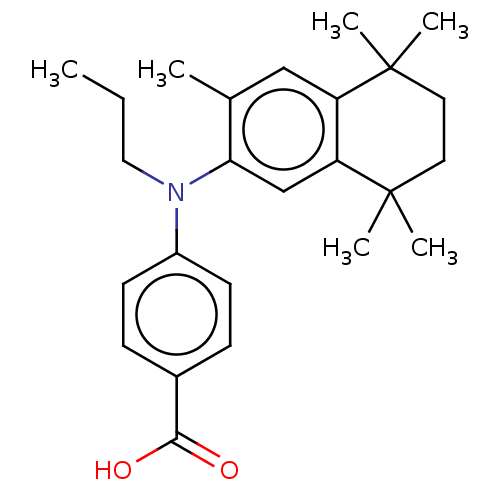
(CHEMBL2321917)Show SMILES CCCN(c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H33NO2/c1-7-14-26(19-10-8-18(9-11-19)23(27)28)22-16-21-20(15-17(22)2)24(3,4)12-13-25(21,5)6/h8-11,15-16H,7,12-14H2,1-6H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Agonist activity at RXR (unknown origin) in presence of RAR agonist Am80 |
Bioorg Med Chem Lett 23: 81-4 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.008
BindingDB Entry DOI: 10.7270/Q20C4ZPG |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50101445
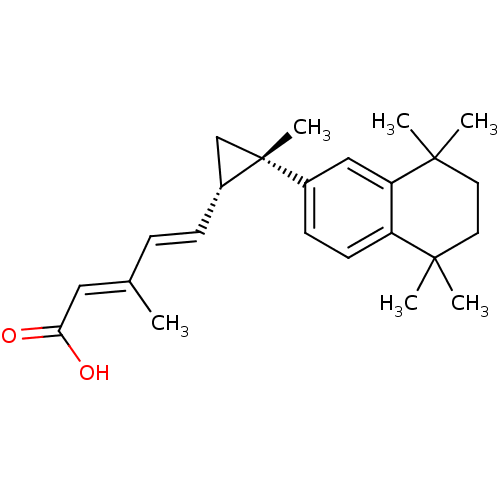
((2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@H]1C[C@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50101444
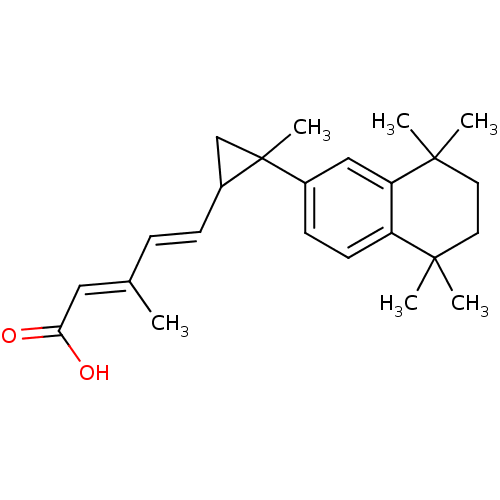
((E)-3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,...)Show SMILES C\C(\C=C\C1CC1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for functional activity in CV-1 cells transfected with an expression vector for retinoid X receptor beta using transactivation as... |
Bioorg Med Chem Lett 6: 213-218 (1996)
Article DOI: 10.1016/0960-894X(95)00588-K
BindingDB Entry DOI: 10.7270/Q2BC3ZHZ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50285919
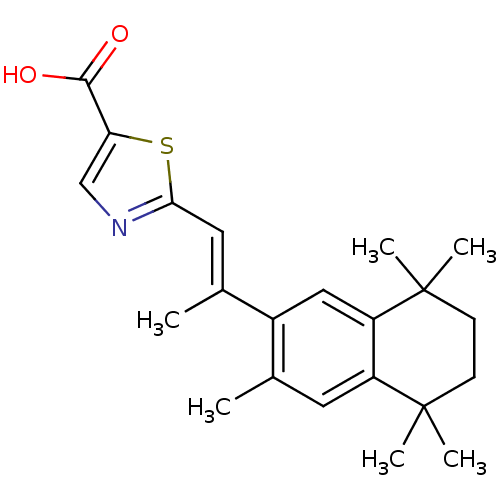
(2-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ncc(s1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C22H27NO2S/c1-13-9-16-17(22(5,6)8-7-21(16,3)4)11-15(13)14(2)10-19-23-12-18(26-19)20(24)25/h9-12H,7-8H2,1-6H3,(H,24,25)/b14-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity for retinoic acid receptor RXR beta in transcriptional activation assay |
Bioorg Med Chem Lett 5: 2729-2734 (1995)
Article DOI: 10.1016/0960-894X(95)00455-3
BindingDB Entry DOI: 10.7270/Q22N527N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00641
BindingDB Entry DOI: 10.7270/Q2K35ZR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50533809
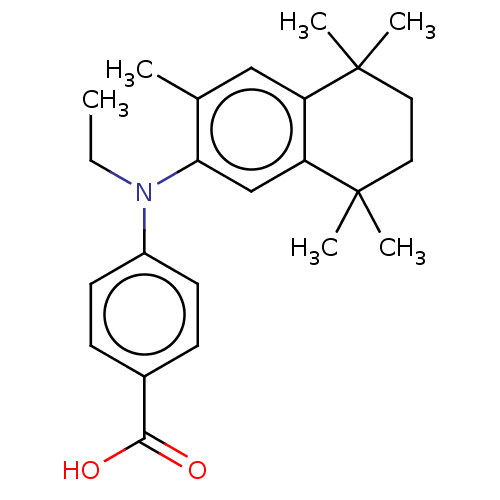
(CHEMBL4547228)Show SMILES CCN(c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H31NO2/c1-7-25(18-10-8-17(9-11-18)22(26)27)21-15-20-19(14-16(21)2)23(3,4)12-13-24(20,5)6/h8-11,14-15H,7,12-13H2,1-6H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052588
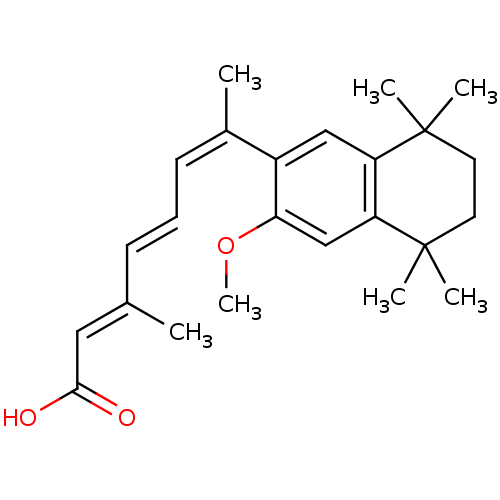
((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...)Show SMILES COc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H32O3/c1-16(13-22(25)26)9-8-10-17(2)18-14-19-20(15-21(18)27-7)24(5,6)12-11-23(19,3)4/h8-10,13-15H,11-12H2,1-7H3,(H,25,26)/b9-8+,16-13+,17-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR beta |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052590
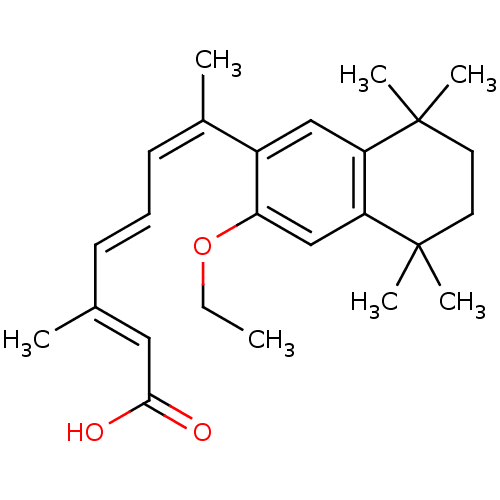
((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES CCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H34O3/c1-8-28-22-16-21-20(24(4,5)12-13-25(21,6)7)15-19(22)18(3)11-9-10-17(2)14-23(26)27/h9-11,14-16H,8,12-13H2,1-7H3,(H,26,27)/b10-9+,17-14+,18-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR beta |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074295
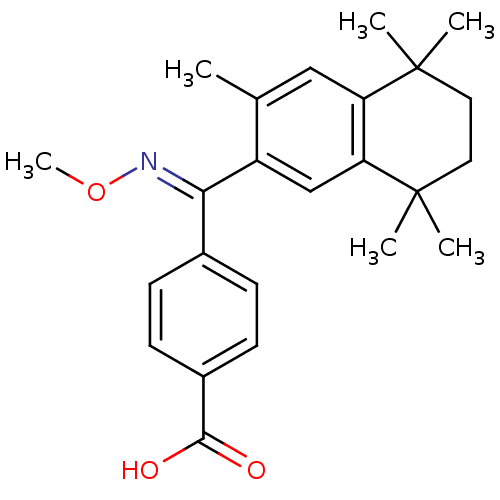
(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074299
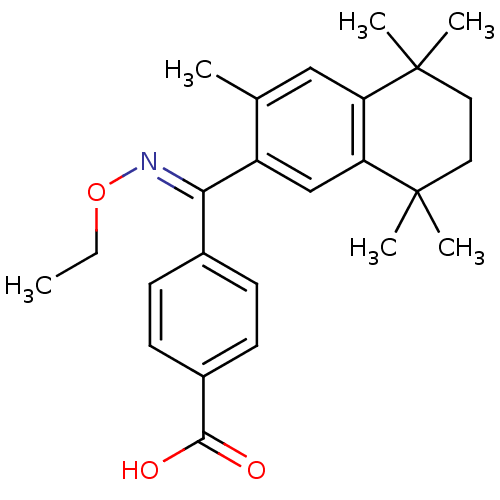
(4-[[(E)-Ethoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7,...)Show SMILES CCO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H31NO3/c1-7-29-26-22(17-8-10-18(11-9-17)23(27)28)19-15-21-20(14-16(19)2)24(3,4)12-13-25(21,5)6/h8-11,14-15H,7,12-13H2,1-6H3,(H,27,28)/b26-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
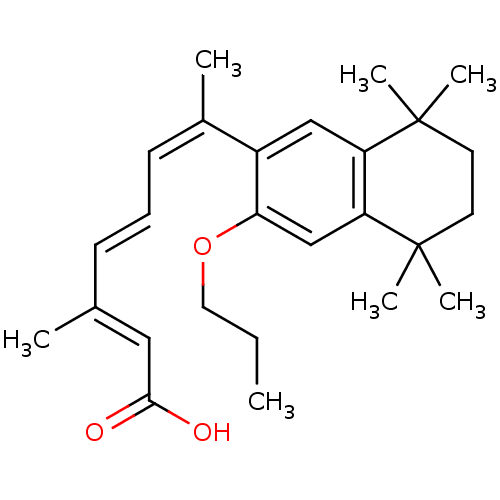
(Homo sapiens (Human)) | BDBM50052589
((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...)Show SMILES CCCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H36O3/c1-8-14-29-23-17-22-21(25(4,5)12-13-26(22,6)7)16-20(23)19(3)11-9-10-18(2)15-24(27)28/h9-11,15-17H,8,12-14H2,1-7H3,(H,27,28)/b10-9+,18-15+,19-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR beta |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50290660
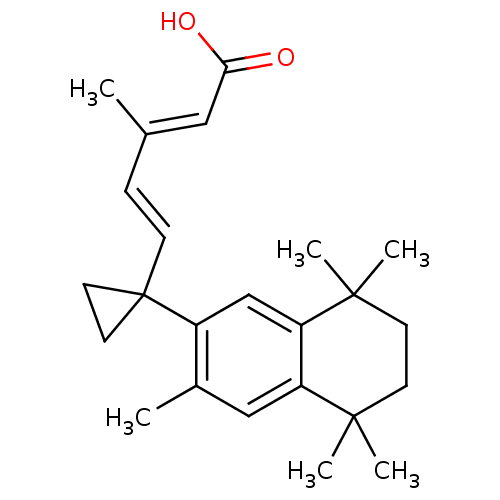
((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(CC1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-24(11-12-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074300
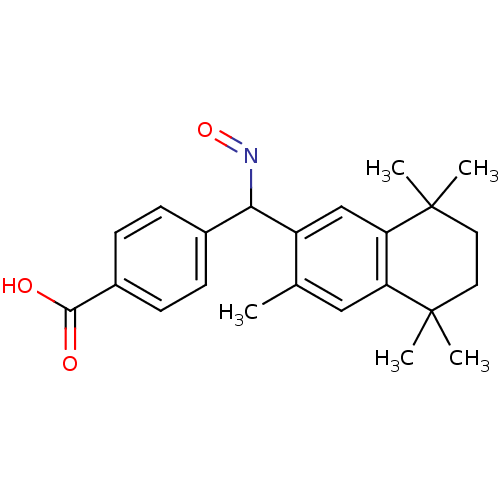
(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50324896
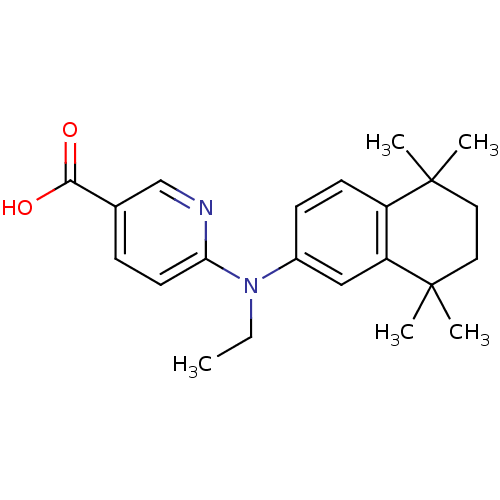
(6-[N-Ethyl(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...)Show SMILES CCN(c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H28N2O2/c1-6-24(19-10-7-15(14-23-19)20(25)26)16-8-9-17-18(13-16)22(4,5)12-11-21(17,2)3/h7-10,13-14H,6,11-12H2,1-5H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50533814
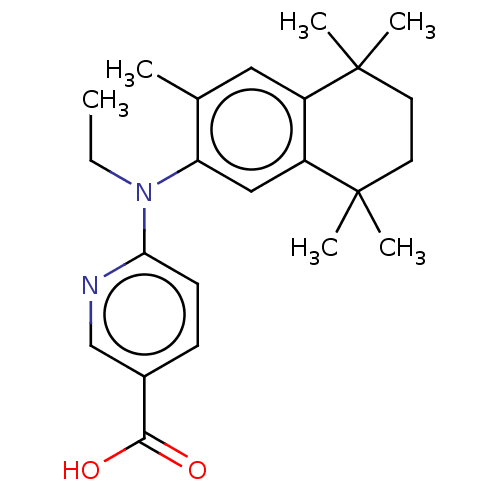
(CHEMBL4459731)Show SMILES CCN(c1ccc(cn1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H30N2O2/c1-7-25(20-9-8-16(14-24-20)21(26)27)19-13-18-17(12-15(19)2)22(3,4)10-11-23(18,5)6/h8-9,12-14H,7,10-11H2,1-6H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50533812
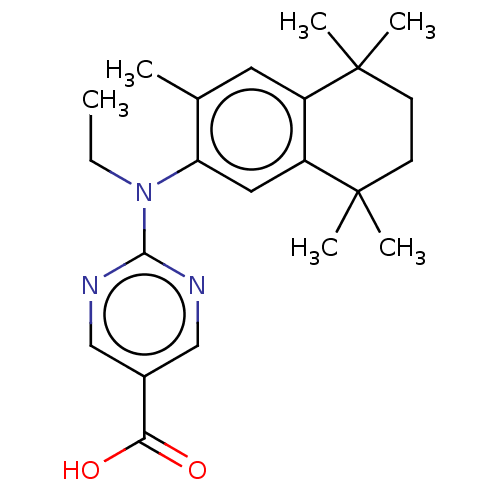
(CHEMBL4545837)Show SMILES CCN(c1ncc(cn1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C22H29N3O2/c1-7-25(20-23-12-15(13-24-20)19(26)27)18-11-17-16(10-14(18)2)21(3,4)8-9-22(17,5)6/h10-13H,7-9H2,1-6H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074306
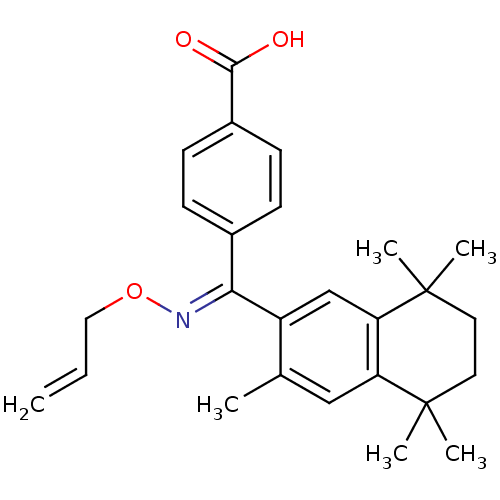
(4-[[(E)-Allyloxyimino]-(3,5,5,8,8-pentamethyl-5,6,...)Show SMILES Cc1cc2c(cc1\C(=N\OCC=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H31NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h7-11,15-16H,1,12-14H2,2-6H3,(H,28,29)/b27-23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50290659
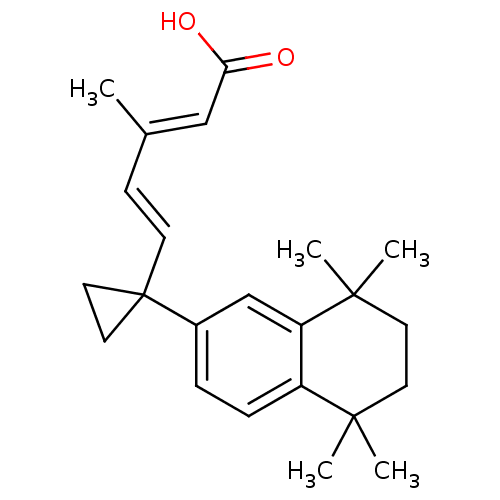
((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...)Show SMILES C\C(\C=C\C1(CC1)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C23H30O2/c1-16(14-20(24)25)8-9-23(12-13-23)17-6-7-18-19(15-17)22(4,5)11-10-21(18,2)3/h6-9,14-15H,10-13H2,1-5H3,(H,24,25)/b9-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50179116
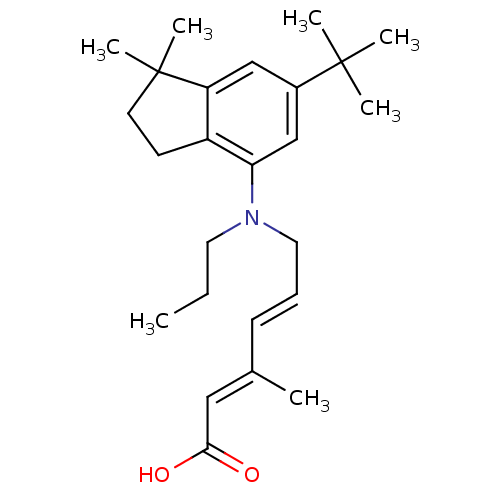
((2E,4E)-6-((6-tert-butyl-1,1-dimethyl-2,3-dihydro-...)Show SMILES CCCN(C\C=C\C(\C)=C\C(O)=O)c1cc(cc2c1CCC2(C)C)C(C)(C)C Show InChI InChI=1S/C25H37NO2/c1-8-13-26(14-9-10-18(2)15-23(27)28)22-17-19(24(3,4)5)16-21-20(22)11-12-25(21,6)7/h9-10,15-17H,8,11-14H2,1-7H3,(H,27,28)/b10-9+,18-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Transactivation of RXRbeta in CV1 cells by cotransfection assay |
Bioorg Med Chem Lett 16: 2352-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.003
BindingDB Entry DOI: 10.7270/Q2S75FW3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50409208

(CHEMBL5289973)Show InChI InChI=1S/C16H20N4S/c21-16(18-10-13-4-2-1-3-5-13)20-8-6-14(7-9-20)15-11-17-12-19-15/h1-5,11-12,14H,6-10H2,(H,17,19)(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat liver Dihydrofolate reductase. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032220

(5-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ccc(s1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H28O2S/c1-14(11-16-7-8-20(26-16)21(24)25)17-13-19-18(12-15(17)2)22(3,4)9-10-23(19,5)6/h7-8,11-13H,9-10H2,1-6H3,(H,24,25)/b14-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity for retinoic acid receptor RXR beta in transcriptional activation assay |
Bioorg Med Chem Lett 5: 2729-2734 (1995)
Article DOI: 10.1016/0960-894X(95)00455-3
BindingDB Entry DOI: 10.7270/Q22N527N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity RXRbeta (unknown origin) |
J Med Chem 62: 2112-2126 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01848
BindingDB Entry DOI: 10.7270/Q2J38X18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
| Assay Description
Agonist activity at RXRbeta (unknown origin) |
J Med Chem 61: 2166-2210 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00315
BindingDB Entry DOI: 10.7270/Q2B56N68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity RXRbeta (unknown origin) |
J Med Chem 62: 2112-2126 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01848
BindingDB Entry DOI: 10.7270/Q2J38X18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat histamine H3 receptor expressed in SK-NM-C cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50409206

(CHEMBL114640)Show InChI InChI=1S/C16H19IN4S/c17-14-3-1-2-12(8-14)9-19-16(22)21-6-4-13(5-7-21)15-10-18-11-20-15/h1-3,8,10-11,13H,4-7,9H2,(H,18,20)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Dihydrofolate reductase of Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074297
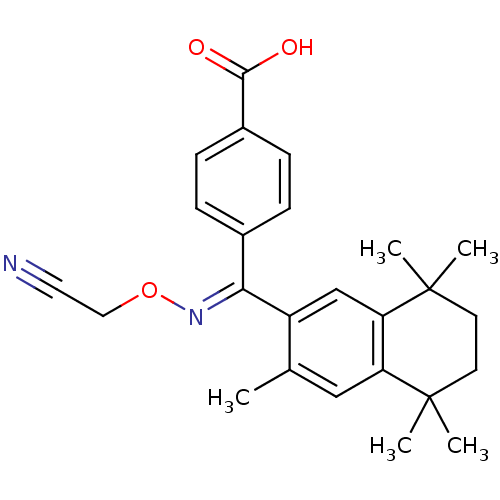
(4-[[(E)-Cyanomethoxyimino]-(3,5,5,8,8-pentamethyl-...)Show SMILES Cc1cc2c(cc1\C(=N\OCC#N)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H28N2O3/c1-16-14-20-21(25(4,5)11-10-24(20,2)3)15-19(16)22(27-30-13-12-26)17-6-8-18(9-7-17)23(28)29/h6-9,14-15H,10-11,13H2,1-5H3,(H,28,29)/b27-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50101446
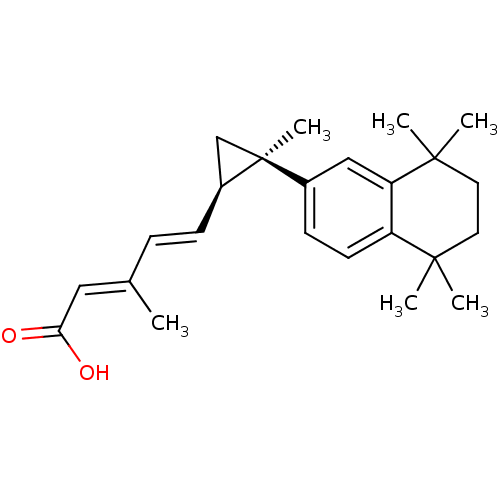
((2E,4E)-3-Methyl-5-[(1R,2R)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@H]1C[C@@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074304
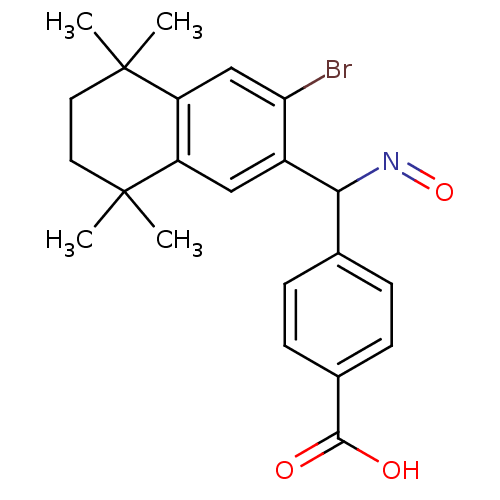
(4-{(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...)Show SMILES CC1(C)CCC(C)(C)c2cc(C(N=O)c3ccc(cc3)C(O)=O)c(Br)cc12 Show InChI InChI=1S/C22H24BrNO3/c1-21(2)9-10-22(3,4)17-12-18(23)15(11-16(17)21)19(24-27)13-5-7-14(8-6-13)20(25)26/h5-8,11-12,19H,9-10H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033076
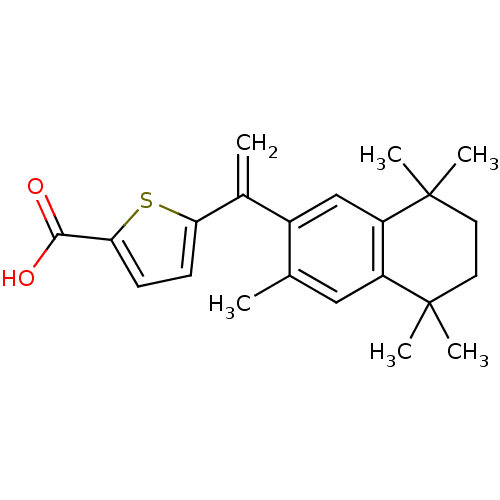
(5-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-nap...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(s1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C22H26O2S/c1-13-11-16-17(22(5,6)10-9-21(16,3)4)12-15(13)14(2)18-7-8-19(25-18)20(23)24/h7-8,11-12H,2,9-10H2,1,3-6H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033065
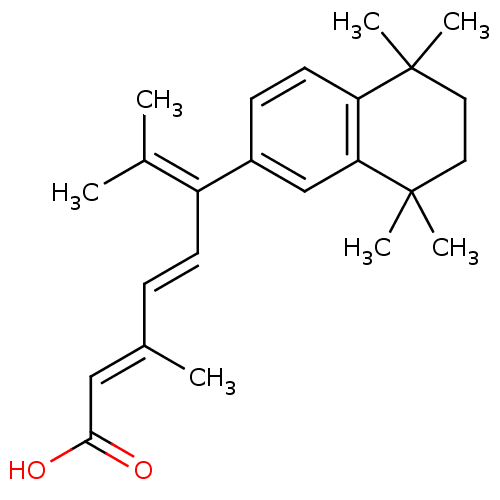
((2E,4E)-3,7-Dimethyl-6-(5,5,8,8-tetramethyl-5,6,7,...)Show SMILES [#6]\[#6](-[#6])=[#6](\[#6]=[#6]\[#6](\[#6])=[#6]\[#6](-[#8])=O)/c1ccc2c(c1)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C24H32O2/c1-16(2)19(10-8-17(3)14-22(25)26)18-9-11-20-21(15-18)24(6,7)13-12-23(20,4)5/h8-11,14-15H,12-13H2,1-7H3,(H,25,26)/b10-8+,17-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50533813
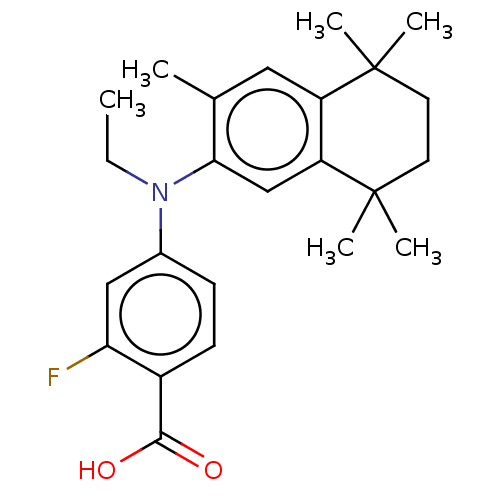
(CHEMBL4476213)Show SMILES CCN(c1ccc(C(O)=O)c(F)c1)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H30FNO2/c1-7-26(16-8-9-17(22(27)28)20(25)13-16)21-14-19-18(12-15(21)2)23(3,4)10-11-24(19,5)6/h8-9,12-14H,7,10-11H2,1-6H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50409207

(CHEMBL5279494)Show InChI InChI=1S/C16H19FN4S/c17-14-3-1-2-12(8-14)9-19-16(22)21-6-4-13(5-7-21)15-10-18-11-20-15/h1-3,8,10-11,13H,4-7,9H2,(H,18,20)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat liver Dihydrofolate reductase. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074307
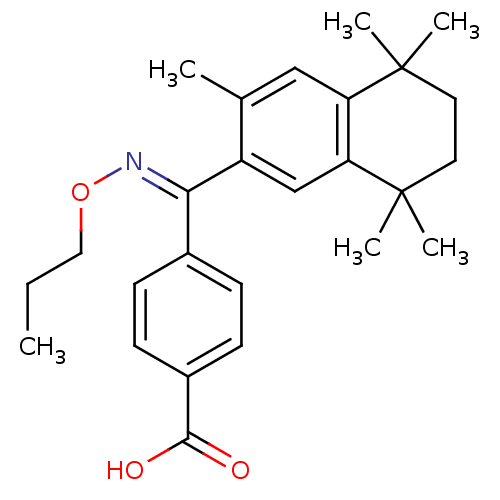
(4-{(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CCCO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H33NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h8-11,15-16H,7,12-14H2,1-6H3,(H,28,29)/b27-23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033079
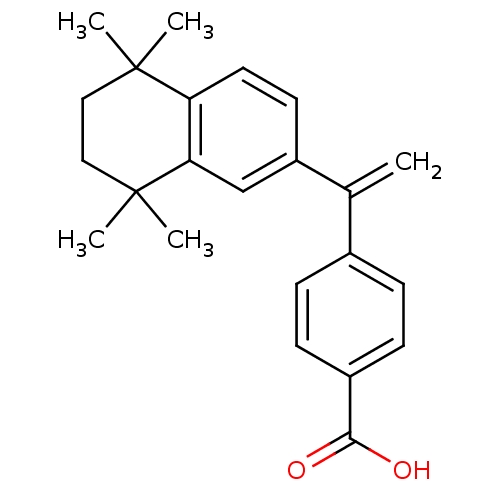
(4-[1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=C)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H26O2/c1-15(16-6-8-17(9-7-16)21(24)25)18-10-11-19-20(14-18)23(4,5)13-12-22(19,2)3/h6-11,14H,1,12-13H2,2-5H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoid X receptor RXR beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50575872
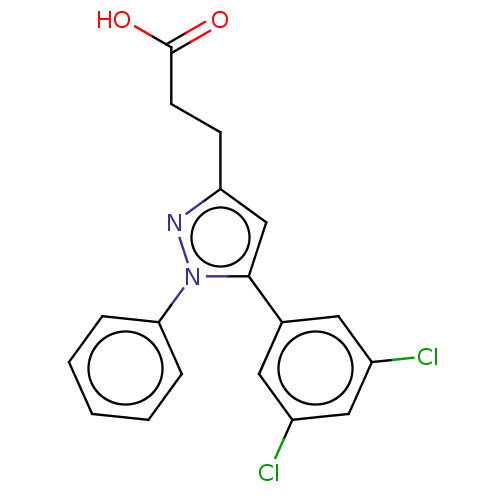
(CHEMBL4873606)Show SMILES OC(=O)CCc1cc(-c2cc(Cl)cc(Cl)c2)n(n1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human RXRbeta transfected in human HEK293T cells co-expressing pFR/pRL-Luc incubated for 14 to 16 hrs by hybrid reporter gene ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00235
BindingDB Entry DOI: 10.7270/Q2D222FF |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50533804
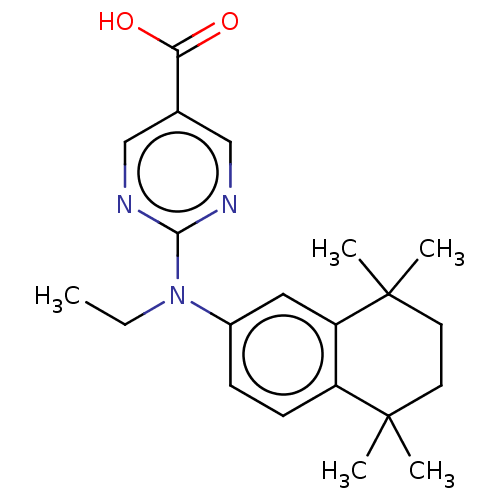
(CHEMBL4574155)Show SMILES CCN(c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ncc(cn1)C(O)=O Show InChI InChI=1S/C21H27N3O2/c1-6-24(19-22-12-14(13-23-19)18(25)26)15-7-8-16-17(11-15)21(4,5)10-9-20(16,2)3/h7-8,11-13H,6,9-10H2,1-5H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033068
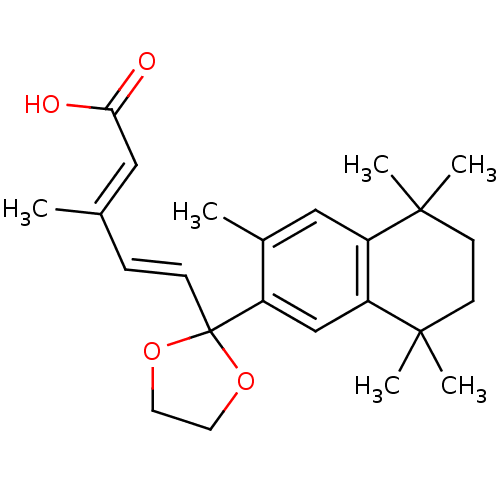
((2E,4E)-3-Methyl-5-[2-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(OCCO1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O4/c1-16(13-21(25)26)7-8-24(27-11-12-28-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoid X receptor RXR beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50575868
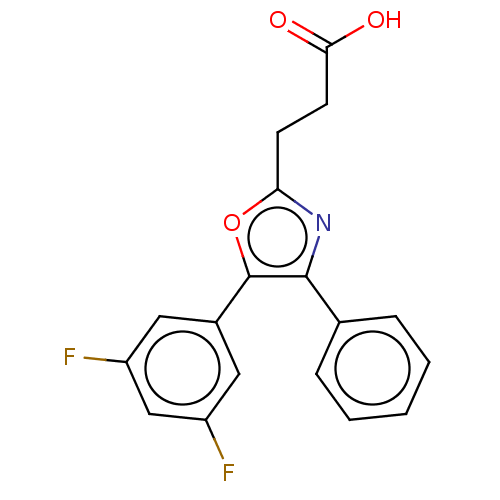
(CHEMBL4865656)Show SMILES OC(=O)CCc1nc(c(o1)-c1cc(F)cc(F)c1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human RXRbeta transfected in human HEK293T cells co-expressing pFR/pRL-Luc incubated for 14 to 16 hrs by hybrid reporter gene ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00235
BindingDB Entry DOI: 10.7270/Q2D222FF |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50575861
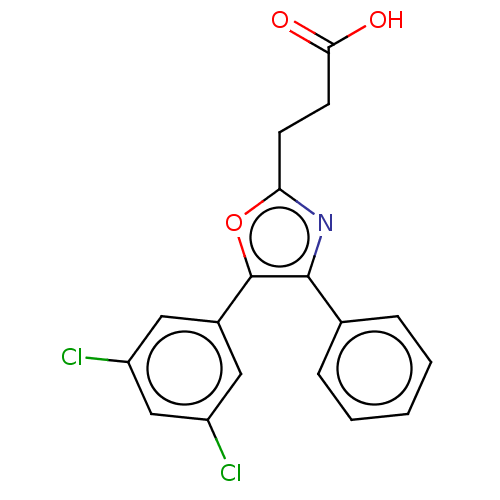
(CHEMBL4874191)Show SMILES OC(=O)CCc1nc(c(o1)-c1cc(Cl)cc(Cl)c1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human RXRbeta transfected in human HEK293T cells co-expressing pFR/pRL-Luc incubated for 14 to 16 hrs by hybrid reporter gene ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00235
BindingDB Entry DOI: 10.7270/Q2D222FF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50179113
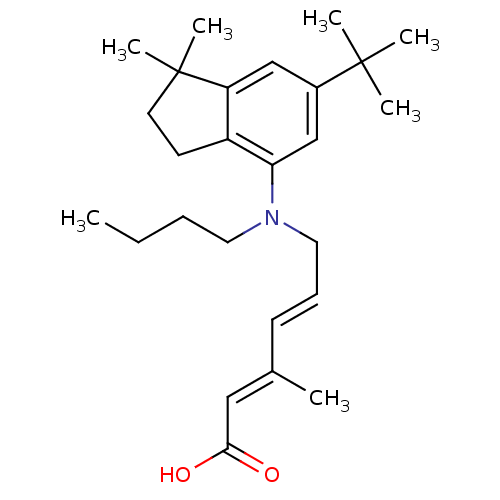
((2E,4E)-6-(butyl(6-tert-butyl-1,1-dimethyl-2,3-dih...)Show SMILES CCCCN(C\C=C\C(\C)=C\C(O)=O)c1cc(cc2c1CCC2(C)C)C(C)(C)C Show InChI InChI=1S/C26H39NO2/c1-8-9-14-27(15-10-11-19(2)16-24(28)29)23-18-20(25(3,4)5)17-22-21(23)12-13-26(22,6)7/h10-11,16-18H,8-9,12-15H2,1-7H3,(H,28,29)/b11-10+,19-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Transactivation of RXRbeta in CV1 cells by cotransfection assay |
Bioorg Med Chem Lett 16: 2352-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.003
BindingDB Entry DOI: 10.7270/Q2S75FW3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Arizona State University
Curated by ChEMBL
| Assay Description
Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs... |
J Med Chem 59: 8924-8940 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00812
BindingDB Entry DOI: 10.7270/Q26M3B90 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50052962
(6-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1Sc1ccc(cn1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C21H25NO2S/c1-13-10-15-16(21(4,5)9-8-20(15,2)3)11-17(13)25-18-7-6-14(12-22-18)19(23)24/h6-7,10-12H,8-9H2,1-5H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR beta |
J Med Chem 39: 3556-63 (1996)
Article DOI: 10.1021/jm960386h
BindingDB Entry DOI: 10.7270/Q2959GNV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074305

(4-[[(E)-2-Amino-ethoxyimino]-(3,5,5,8,8-pentamethy...)Show SMILES Cc1cc2c(cc1\C(=N\OCCN)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H32N2O3/c1-16-14-20-21(25(4,5)11-10-24(20,2)3)15-19(16)22(27-30-13-12-26)17-6-8-18(9-7-17)23(28)29/h6-9,14-15H,10-13,26H2,1-5H3,(H,28,29)/b27-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-beta |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data