 Found 278 hits of ki data for polymerid = 5102
Found 278 hits of ki data for polymerid = 5102 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 35: 725-34 (1996)
Article DOI: 10.1016/0028-3908(96)84644-2
BindingDB Entry DOI: 10.7270/Q237777N |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 35: 725-34 (1996)
Article DOI: 10.1016/0028-3908(96)84644-2
BindingDB Entry DOI: 10.7270/Q237777N |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 35: 725-34 (1996)
Article DOI: 10.1016/0028-3908(96)84644-2
BindingDB Entry DOI: 10.7270/Q237777N |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM86311
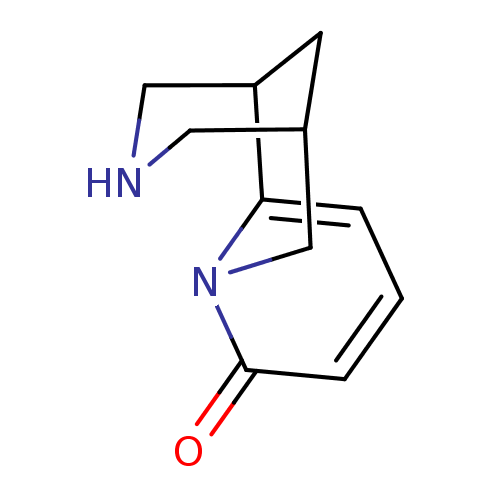
(CAS_485-35-8 | Cytisine | Cytisine-(-) | NSC_22407...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50107863
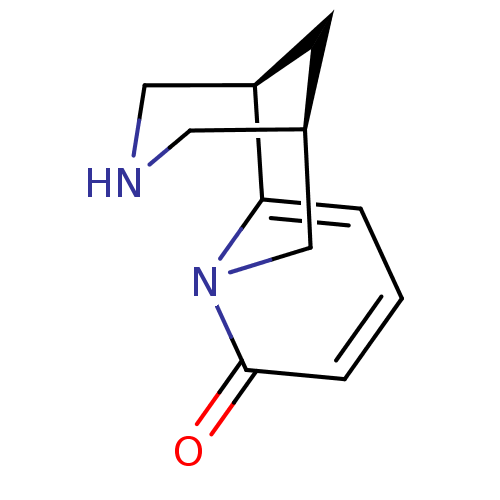
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50261762
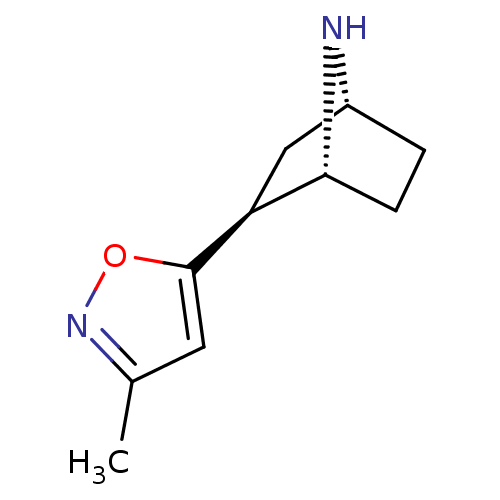
((+/-)-exo-2-(3-methylisoxazol-5-yl)-7-aza-bicyclo[...)Show SMILES Cc1cc(on1)[C@H]1C[C@H]2CC[C@H]1N2 |r,THB:3:6:9.10:12| Show InChI InChI=1S/C10H14N2O/c1-6-4-10(13-12-6)8-5-7-2-3-9(8)11-7/h4,7-9,11H,2-3,5H2,1H3/t7-,8+,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR (unknown origin) |
Bioorg Med Chem Lett 18: 4651-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.016
BindingDB Entry DOI: 10.7270/Q20C4VKW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50023330
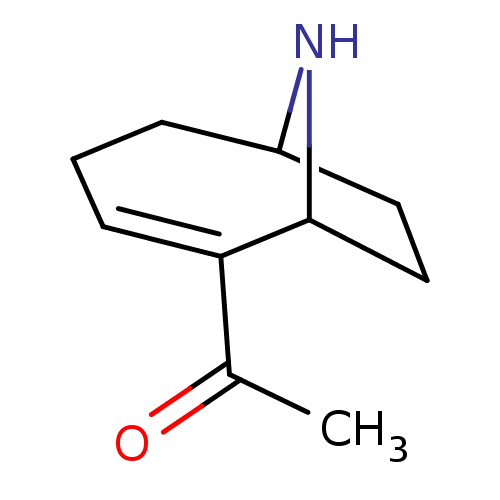
(1-(9-Aza-bicyclo[4.2.1]non-2-en-2-yl)-ethanone | 1...)Show InChI InChI=1S/C10H15NO/c1-7(12)9-4-2-3-8-5-6-10(9)11-8/h4,8,10-11H,2-3,5-6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 35: 725-34 (1996)
Article DOI: 10.1016/0028-3908(96)84644-2
BindingDB Entry DOI: 10.7270/Q237777N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50261762

((+/-)-exo-2-(3-methylisoxazol-5-yl)-7-aza-bicyclo[...)Show SMILES Cc1cc(on1)[C@H]1C[C@H]2CC[C@H]1N2 |r,THB:3:6:9.10:12| Show InChI InChI=1S/C10H14N2O/c1-6-4-10(13-12-6)8-5-7-2-3-9(8)11-7/h4,7-9,11H,2-3,5H2,1H3/t7-,8+,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [3H]cystisine from alpha4beta2 nAChR (unknown origin) |
Bioorg Med Chem Lett 18: 4651-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.016
BindingDB Entry DOI: 10.7270/Q20C4VKW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397942
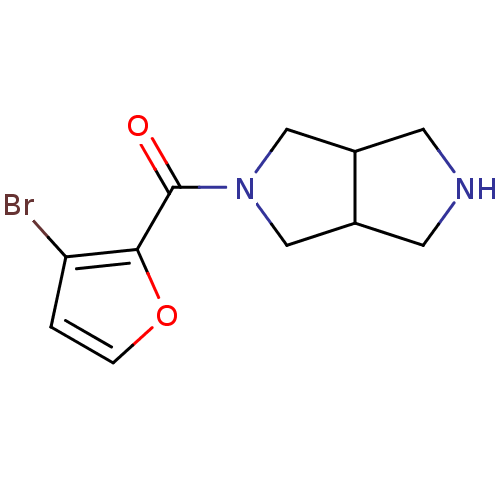
(CHEMBL2179534)Show InChI InChI=1S/C11H13BrN2O2/c12-9-1-2-16-10(9)11(15)14-5-7-3-13-4-8(7)6-14/h1-2,7-8,13H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | -11.9 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141885

(US8921410, Table 2 Compound 20)Show InChI InChI=1S/C12H14Br2N2O2/c13-9-2-10(18-11(9)14)12(17)16-5-7-1-8(6-16)4-15-3-7/h2,7-8,15H,1,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.60 | -11.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397929

(CHEMBL2179547)Show InChI InChI=1S/C12H15BrN2O2/c13-10-2-11(17-7-10)12(16)15-5-8-1-9(6-15)4-14-3-8/h2,7-9,14H,1,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | -11.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50035398
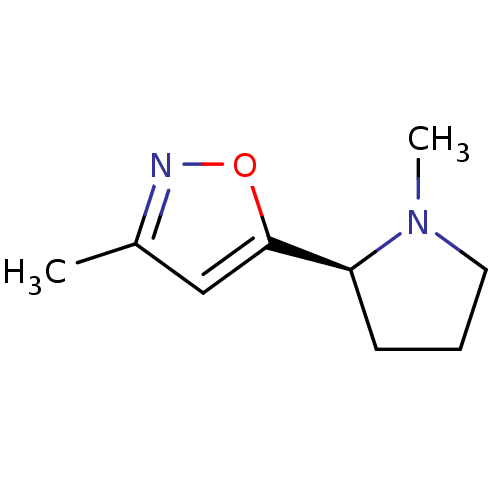
((S)-1-Methyl-2-(3-methyl-isoxazol-5-yl)-pyrrolidin...)Show InChI InChI=1S/C9H14N2O/c1-7-6-9(12-10-7)8-4-3-5-11(8)2/h6,8H,3-5H2,1-2H3/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50047021
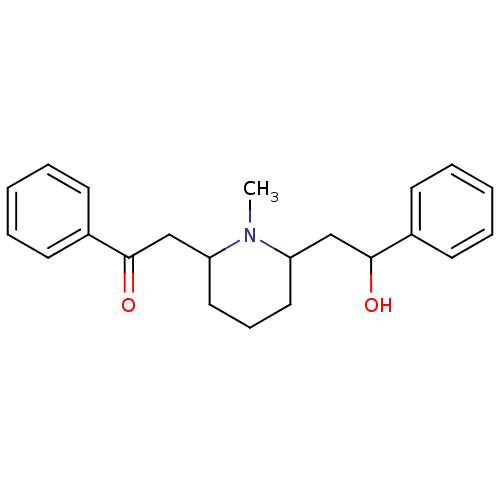
(2-(6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2...)Show InChI InChI=1S/C22H27NO2/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18/h2-7,9-12,19-21,24H,8,13-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50047021

(2-(6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2...)Show InChI InChI=1S/C22H27NO2/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18/h2-7,9-12,19-21,24H,8,13-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397888
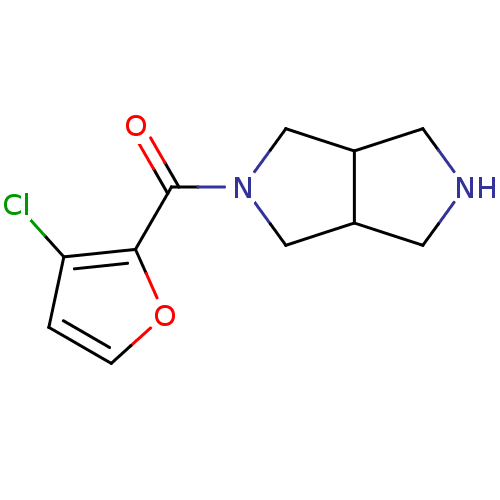
(CHEMBL2179531)Show InChI InChI=1S/C11H13ClN2O2/c12-9-1-2-16-10(9)11(15)14-5-7-3-13-4-8(7)6-14/h1-2,7-8,13H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 2.40 | -11.6 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50023330

(1-(9-Aza-bicyclo[4.2.1]non-2-en-2-yl)-ethanone | 1...)Show InChI InChI=1S/C10H15NO/c1-7(12)9-4-2-3-8-5-6-10(9)11-8/h4,8,10-11H,2-3,5-6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50107863

((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM85224
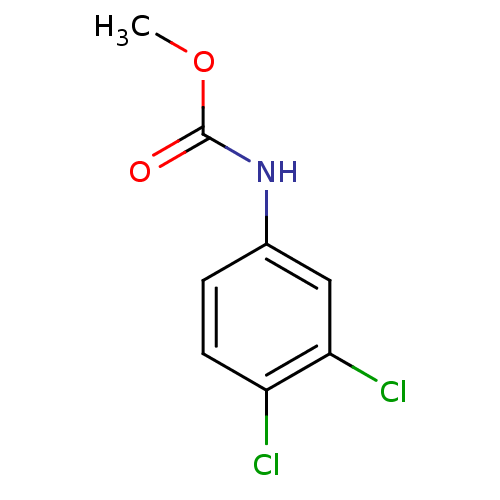
(CAS_1918-18-9 | MCC | SWEP)Show InChI InChI=1S/C8H7Cl2NO2/c1-13-8(12)11-5-2-3-6(9)7(10)4-5/h2-4H,1H3,(H,11,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50023330

(1-(9-Aza-bicyclo[4.2.1]non-2-en-2-yl)-ethanone | 1...)Show InChI InChI=1S/C10H15NO/c1-7(12)9-4-2-3-8-5-6-10(9)11-8/h4,8,10-11H,2-3,5-6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397936

(CHEMBL2179540)Show InChI InChI=1S/C12H15FN2O2/c13-11-2-1-10(17-11)12(16)15-6-8-3-9(7-15)5-14-4-8/h1-2,8-9,14H,3-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 4.60 | -11.2 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141867

(US8921410, Table 1 Compound 33)Show InChI InChI=1S/C11H12Br2N2O2/c12-8-1-9(17-10(8)13)11(16)15-4-6-2-14-3-7(6)5-15/h1,6-7,14H,2-5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4.90 | -11.2 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141887
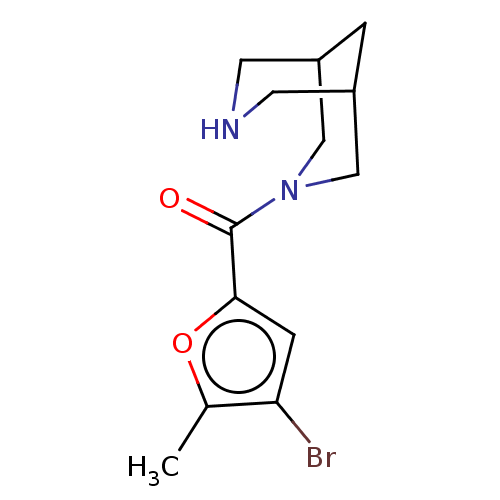
(US8921410, Table 2 Compound 25)Show InChI InChI=1S/C13H17BrN2O2/c1-8-11(14)3-12(18-8)13(17)16-6-9-2-10(7-16)5-15-4-9/h3,9-10,15H,2,4-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 5.20 | -11.1 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397932
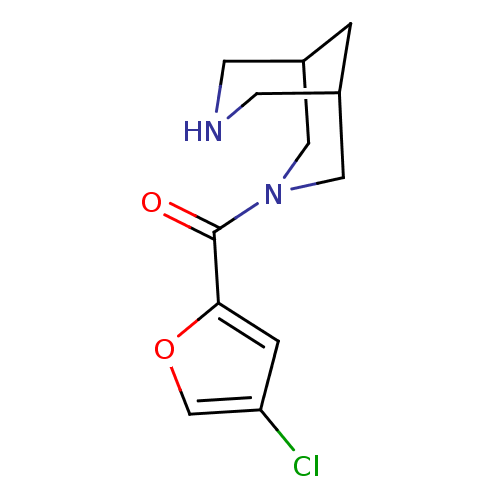
(CHEMBL2179544)Show InChI InChI=1S/C12H15ClN2O2/c13-10-2-11(17-7-10)12(16)15-5-8-1-9(6-15)4-14-3-8/h2,7-9,14H,1,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 5.20 | -11.1 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397933
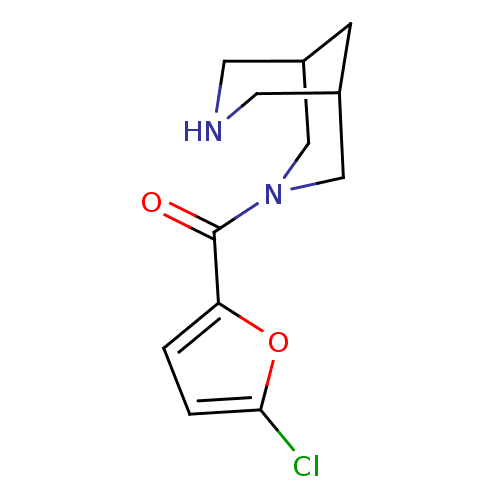
(CHEMBL2179543)Show InChI InChI=1S/C12H15ClN2O2/c13-11-2-1-10(17-11)12(16)15-6-8-3-9(7-15)5-14-4-8/h1-2,8-9,14H,3-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 7.30 | -10.9 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50261766
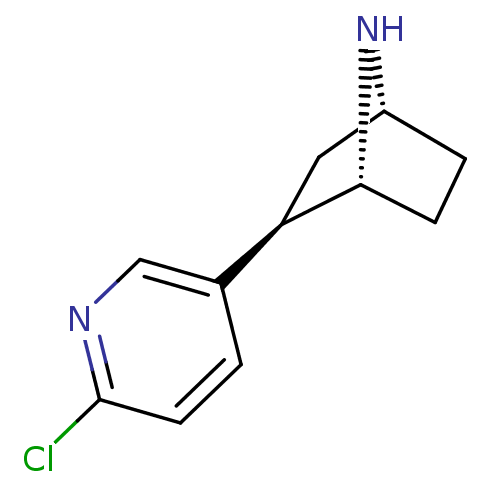
((+/-)-exo-(1R,2R,4R)-2-(6-chloropyridin-3-yl)-7-az...)Show SMILES Clc1ccc(cn1)[C@H]1C[C@H]2CC[C@H]1N2 |r,THB:4:7:10.11:13| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8-,9-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Milano
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR (unknown origin) |
Bioorg Med Chem Lett 18: 4651-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.016
BindingDB Entry DOI: 10.7270/Q20C4VKW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 950-62 (1998)
BindingDB Entry DOI: 10.7270/Q2B856PT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141891
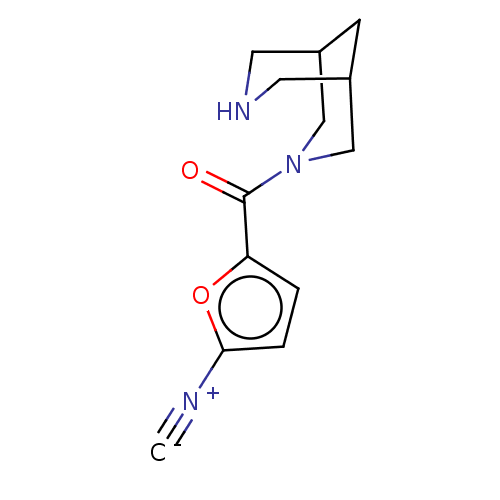
(US8921410, Table 2 Compound 30)Show InChI InChI=1S/C13H15N3O2/c1-14-12-3-2-11(18-12)13(17)16-7-9-4-10(8-16)6-15-5-9/h2-3,9-10,15H,4-8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 8.70 | -10.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397913
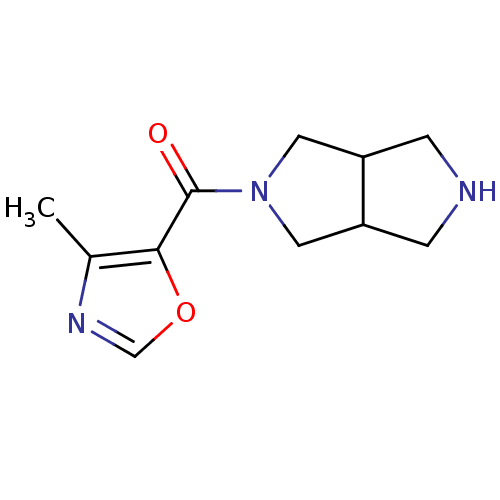
(CHEMBL2179516)Show InChI InChI=1S/C11H15N3O2/c1-7-10(16-6-13-7)11(15)14-4-8-2-12-3-9(8)5-14/h6,8-9,12H,2-5H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 9.20 | -10.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397889

(CHEMBL2179530)Show InChI InChI=1S/C11H13ClN2O2/c12-9-1-10(16-6-9)11(15)14-4-7-2-13-3-8(7)5-14/h1,6-8,13H,2-5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 9.70 | -10.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397899

(CHEMBL2179967)Show InChI InChI=1S/C12H16N2O2/c15-12(11-2-1-3-16-11)14-7-9-4-10(8-14)6-13-5-9/h1-3,9-10,13H,4-8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 9.90 | -10.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141886
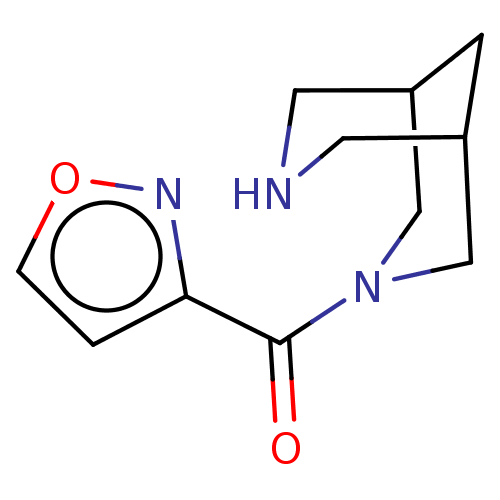
(US8921410, Table 2 Compound 24)Show InChI InChI=1S/C11H15N3O2/c15-11(10-1-2-16-13-10)14-6-8-3-9(7-14)5-12-4-8/h1-2,8-9,12H,3-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 10 | -10.8 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50061567
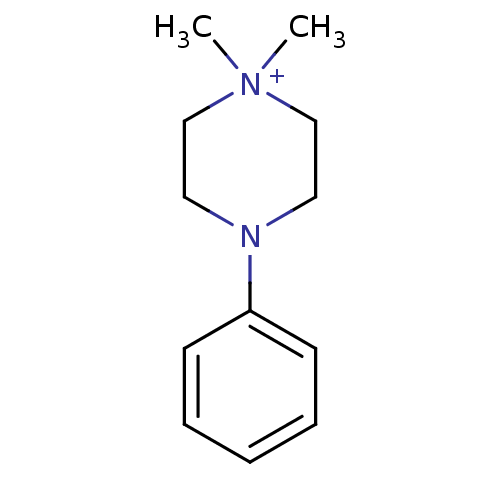
(1,1-Dimethyl-4-phenyl-piperazin-1-ium | CHEMBL1347...)Show InChI InChI=1S/C12H19N2/c1-14(2)10-8-13(9-11-14)12-6-4-3-5-7-12/h3-7H,8-11H2,1-2H3/q+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 276: 289-97 (1996)
BindingDB Entry DOI: 10.7270/Q2GM85T1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50254538

(3-(dimethylamino)butyl methylcarbamate | CHEMBL466...)Show InChI InChI=1S/C8H18N2O2/c1-7(10(3)4)5-6-12-8(11)9-2/h7H,5-6H2,1-4H3,(H,9,11) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Copenhagen University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR (unknown origin) expressed in HEK293 cells |
Bioorg Med Chem Lett 19: 87-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.011
BindingDB Entry DOI: 10.7270/Q2571BV9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM85207
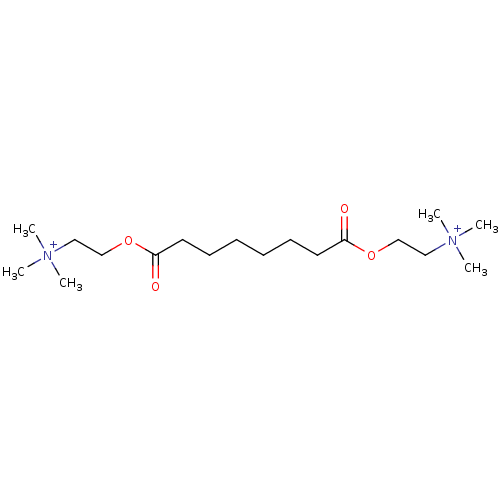
(CAS_123990 | NSC_123990 | Suberyldicholine)Show SMILES C[N+](C)(C)CCOC(=O)CCCCCCC(=O)OCC[N+](C)(C)C Show InChI InChI=1S/C18H38N2O4/c1-19(2,3)13-15-23-17(21)11-9-7-8-10-12-18(22)24-16-14-20(4,5)6/h7-16H2,1-6H3/q+2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1283-94 (2003)
Article DOI: 10.1124/mol.64.6.1283
BindingDB Entry DOI: 10.7270/Q2GF0S2V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141856
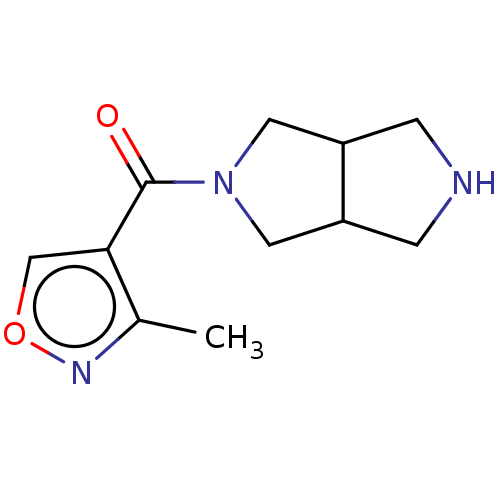
(US8921410, Table 1 Compound 20)Show InChI InChI=1S/C11H15N3O2/c1-7-10(6-16-13-7)11(15)14-4-8-2-12-3-9(8)5-14/h6,8-9,12H,2-5H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 14 | -10.6 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM141868
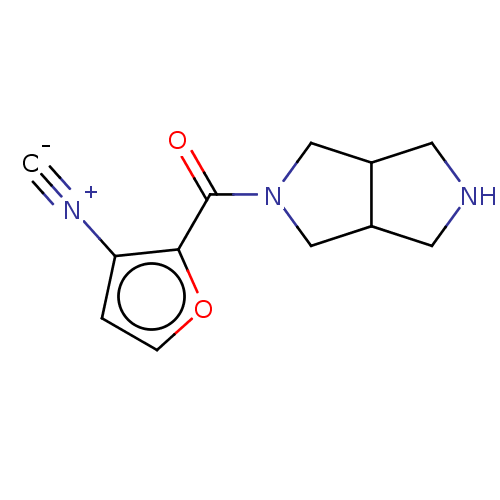
(US8921410, Table 1 Compound 35)Show InChI InChI=1S/C12H13N3O2/c1-13-10-2-3-17-11(10)12(16)15-6-8-4-14-5-9(8)7-15/h2-3,8-9,14H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 14 | -10.6 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397935
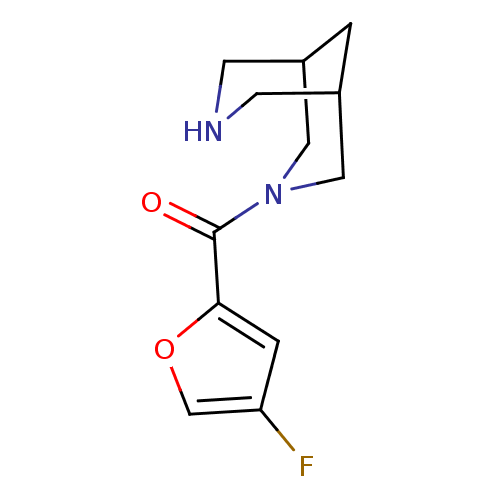
(CHEMBL2179541)Show InChI InChI=1S/C12H15FN2O2/c13-10-2-11(17-7-10)12(16)15-5-8-1-9(6-15)4-14-3-8/h2,7-9,14H,1,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 14 | -10.6 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
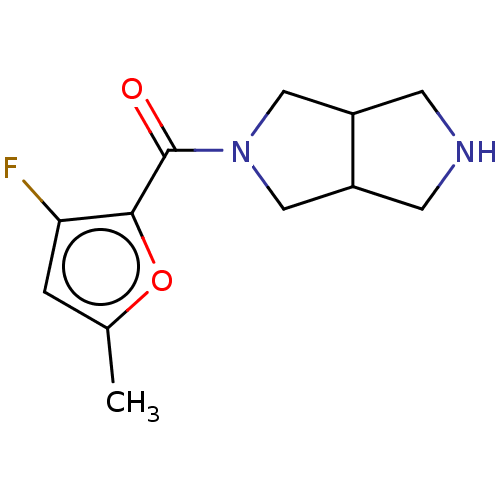
(Homo sapiens (Human)) | BDBM141872
(US8921410, Table 1 Compound 41)Show InChI InChI=1S/C12H15FN2O2/c1-7-2-10(13)11(17-7)12(16)15-5-8-3-14-4-9(8)6-15/h2,8-9,14H,3-6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 14 | -10.6 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50397893
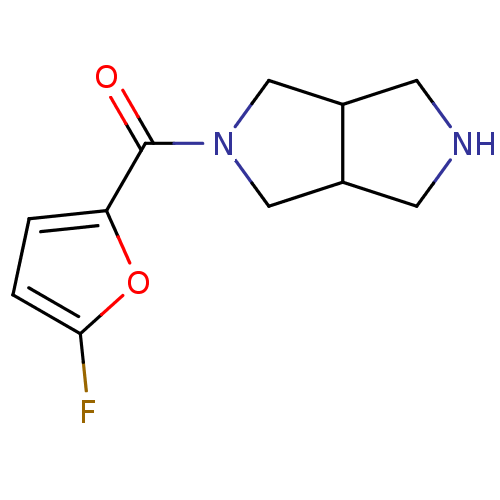
(CHEMBL2179526)Show InChI InChI=1S/C11H13FN2O2/c12-10-2-1-9(16-10)11(15)14-5-7-3-13-4-8(7)6-14/h1-2,7-8,13H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 16 | -10.5 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc.
US Patent
| Assay Description
The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... |
US Patent US8921410 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28PT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data