

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM98660 (US8497385, 18C) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Binding affinity of protein kinase C (PKC). | US Patent US8497385 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM98659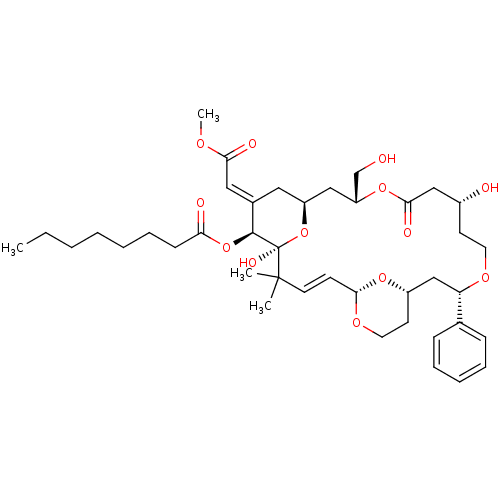 (US8497385, 19B.0) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Binding affinity of protein kinase C (PKC). | US Patent US8497385 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM50282632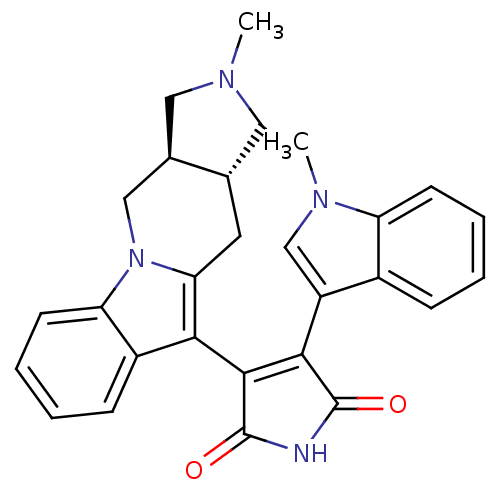 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM98661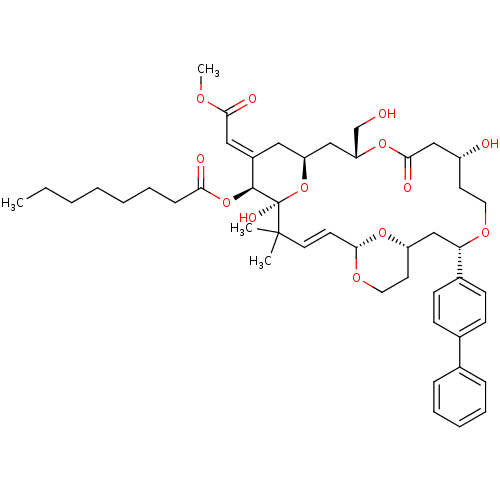 (US8497385, 19B.1) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Binding affinity of protein kinase C (PKC). | US Patent US8497385 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM98662 (US8497385, 19B.2) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Binding affinity of protein kinase C (PKC). | US Patent US8497385 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM98663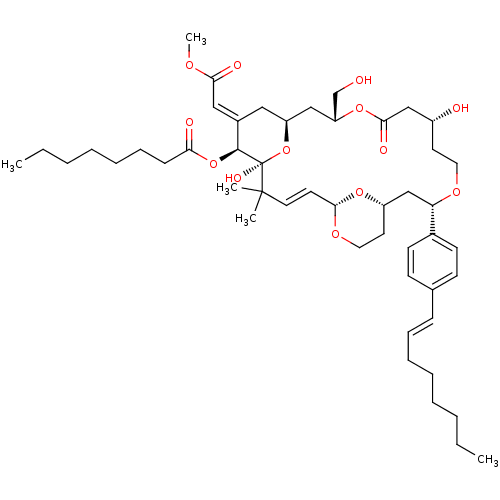 (US8497385, 19B.3) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Binding affinity of protein kinase C (PKC). | US Patent US8497385 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM50282633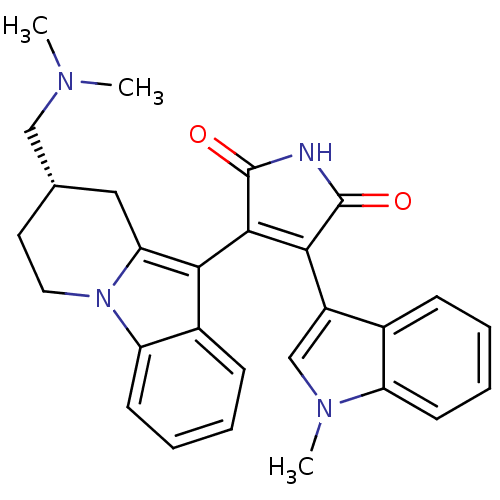 ((S)-3-(8-((dimethylamino)methyl)-6,7,8,9-tetrahydr...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM98664 (US8497385, 19B.4) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Binding affinity of protein kinase C (PKC). | US Patent US8497385 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92971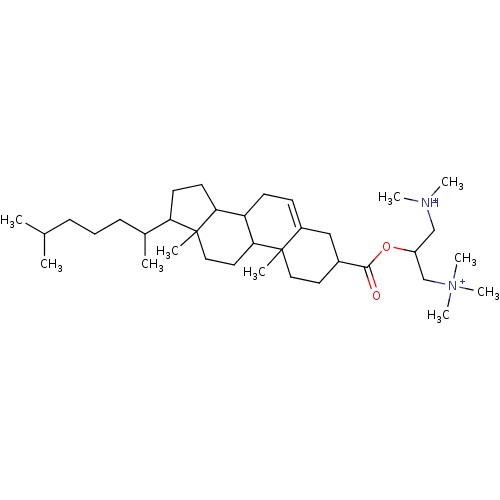 (Amphiphile, VI) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92969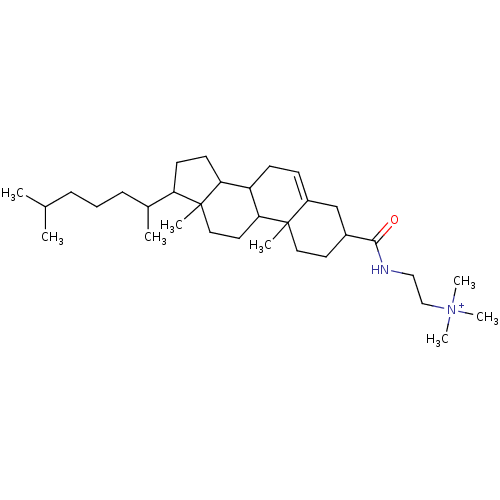 (Amphiphile, IV) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92976 (Amphiphile, XI) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM50009723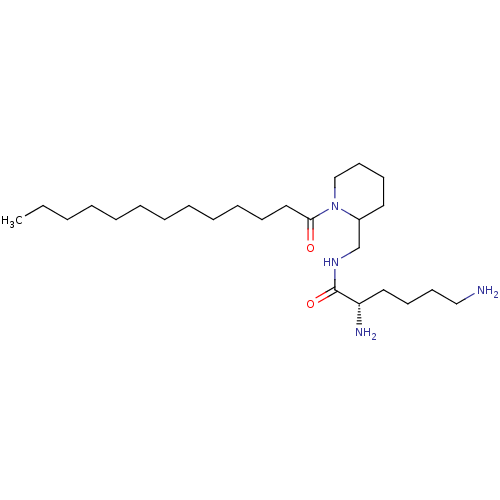 (2,6-Diamino-hexanoic acid (1-tridecanoyl-piperidin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Effect of PMA on inhibition of PKC alpha (Protein kinase C) | J Med Chem 34: 2928-31 (1991) BindingDB Entry DOI: 10.7270/Q2KH0NZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM50009723 (2,6-Diamino-hexanoic acid (1-tridecanoyl-piperidin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Effect of PMA on inhibition of PKC alpha (Protein kinase C) | J Med Chem 34: 2928-31 (1991) BindingDB Entry DOI: 10.7270/Q2KH0NZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92978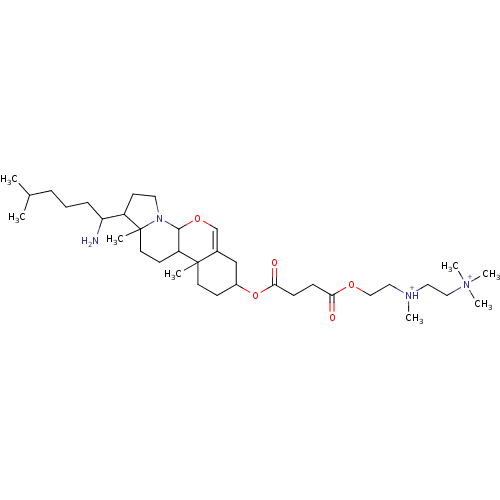 (Amphiphile, XIII) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92979 (Amphiphile, XVI) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92980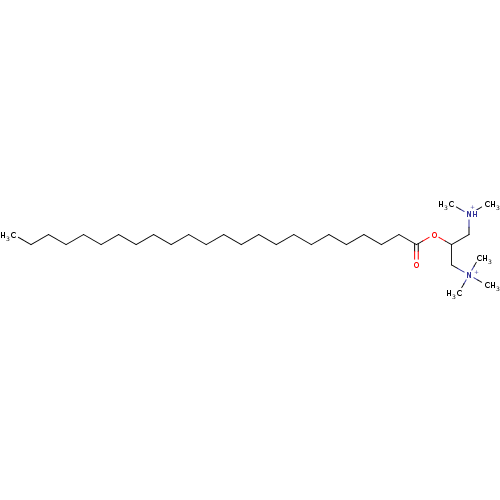 (Amphiphile, XVII) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92974 (Amphiphile, IX) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92977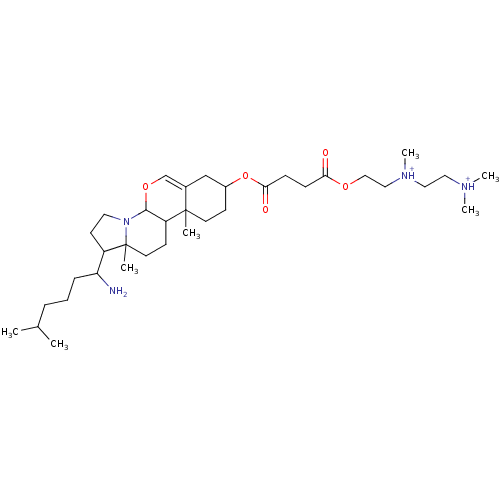 (Amphiphile, XII) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92973 (Amphiphile, VIII) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92972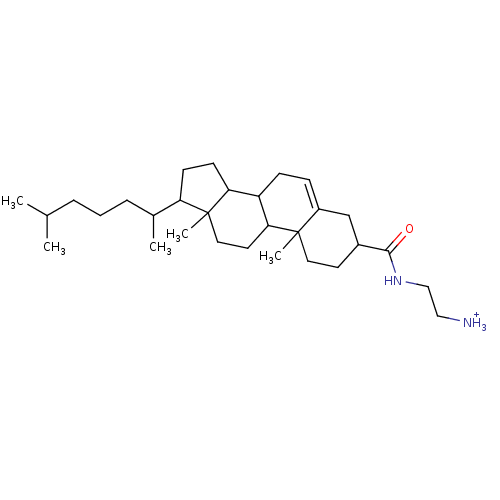 (Amphiphile, VII) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.46E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92968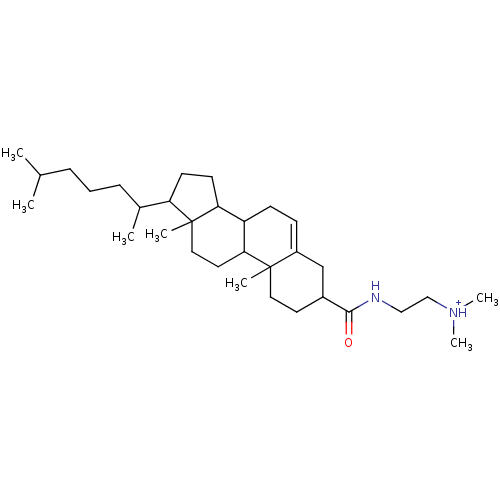 (Amphiphile, III) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92975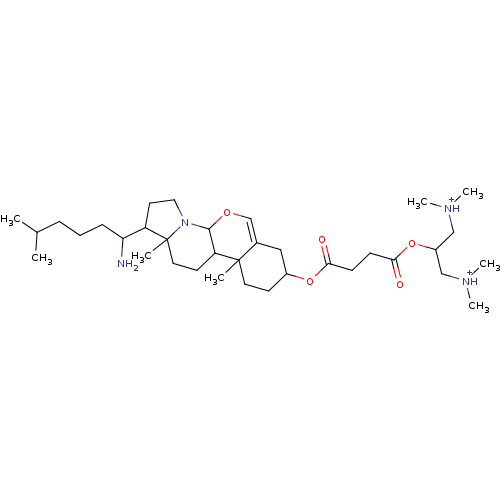 (Amphiphile, X) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.08E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM92970 (Amphiphile, V) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.43E+5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
McMaster University | Assay Description The triton X-100 assay previously described by Bell and co-workers were used to measure enzyme activity. | Biochemistry 31: 9025-30 (1992) Article DOI: 10.1021/bi00152a045 BindingDB Entry DOI: 10.7270/Q2W66JCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||