

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398475 (CHEMBL2179131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398472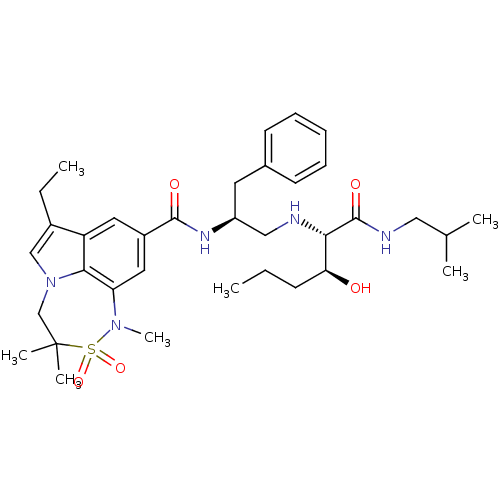 (CHEMBL2179138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16254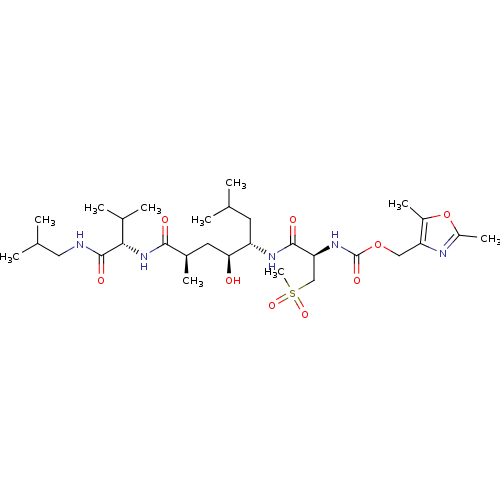 ((2,5-dimethyl-1,3-oxazol-4-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | -14.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
Purdue University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Am Chem Soc 128: 5310-1 (2006) Article DOI: 10.1021/ja058636j BindingDB Entry DOI: 10.7270/Q26W98BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16254 ((2,5-dimethyl-1,3-oxazol-4-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of BACE1 | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16254 ((2,5-dimethyl-1,3-oxazol-4-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant memapsin 2 | J Med Chem 52: 2163-76 (2009) Article DOI: 10.1021/jm900064c BindingDB Entry DOI: 10.7270/Q2KS6RF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50231938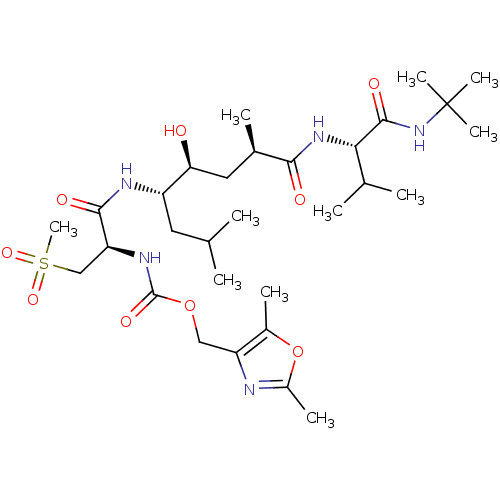 ((2,5-dimethyloxazol-4-yl)methyl (R)-1-((4S,5S,7R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to recombinant memapsin 2 | Bioorg Med Chem Lett 18: 1031-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.028 BindingDB Entry DOI: 10.7270/Q2513XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070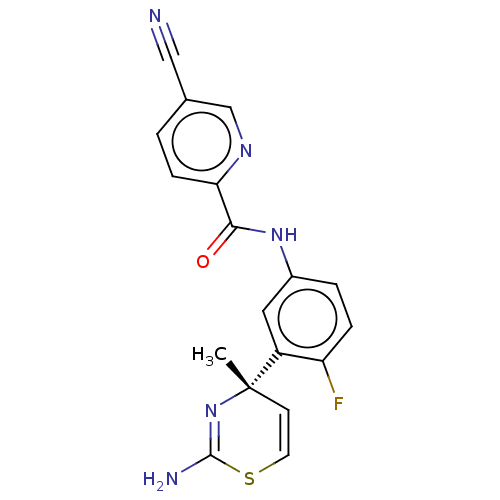 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579806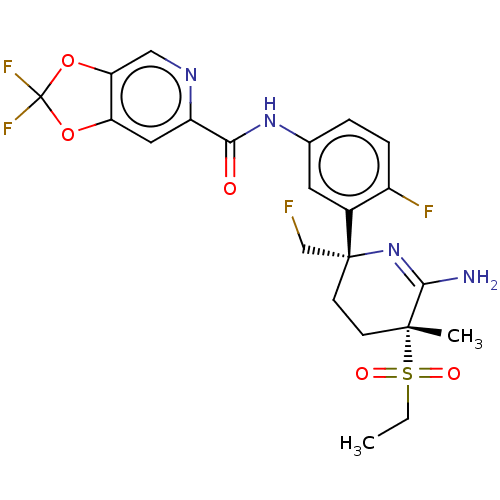 (CHEMBL5092328) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00935 BindingDB Entry DOI: 10.7270/Q2V69PFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50042296 (CHEMBL3352906) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 (unknown origin) | Bioorg Med Chem Lett 25: 668-72 (2015) Article DOI: 10.1016/j.bmcl.2014.11.087 BindingDB Entry DOI: 10.7270/Q2N58P0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16253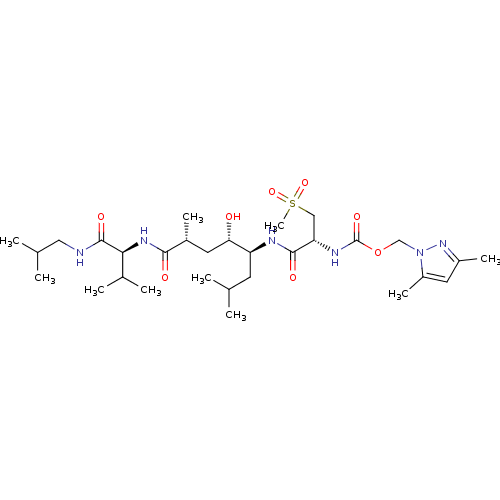 ((3,5-dimethyl-1H-pyrazol-1-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | -13.5 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
Purdue University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Am Chem Soc 128: 5310-1 (2006) Article DOI: 10.1021/ja058636j BindingDB Entry DOI: 10.7270/Q26W98BN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16253 ((3,5-dimethyl-1H-pyrazol-1-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant memapsin 2 | J Med Chem 52: 2163-76 (2009) Article DOI: 10.1021/jm900064c BindingDB Entry DOI: 10.7270/Q2KS6RF1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50155999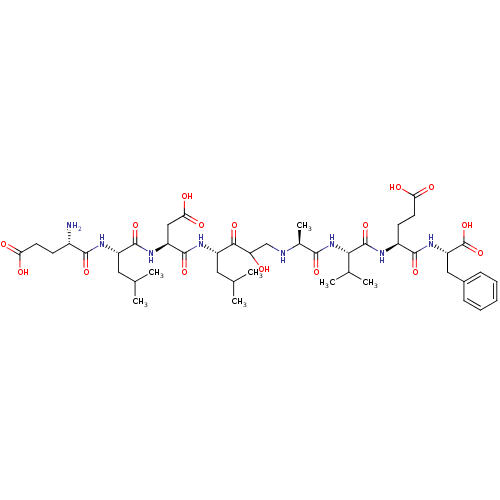 (CHEMBL363255 | Glu-Leu-Asp-Leu-(CHOH-CH2)-Ala-Ala-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zürich Curated by ChEMBL | Assay Description Binding affinity towards Beta-secretase determined using continuum electrostatics solvation | J Med Chem 47: 5791-7 (2004) Article DOI: 10.1021/jm049726m BindingDB Entry DOI: 10.7270/Q2NG4Q3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579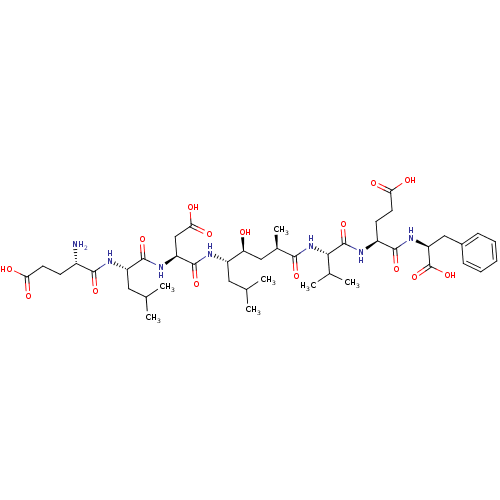 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 ectodomain (1 to 460 amino acids) assessed as inhibition of proteolytic cleavage of Rhodamine-EVNLDAEFK-Quenche... | J Med Chem 54: 3081-5 (2011) Article DOI: 10.1021/jm101568y BindingDB Entry DOI: 10.7270/Q20C4W7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16253 ((3,5-dimethyl-1H-pyrazol-1-yl)methyl N-[(1R)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 (unknown origin) | Bioorg Med Chem Lett 25: 668-72 (2015) Article DOI: 10.1016/j.bmcl.2014.11.087 BindingDB Entry DOI: 10.7270/Q2N58P0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biologia Experimental e Tecnol£gica Curated by ChEMBL | Assay Description Inhibition of active BACE1 (unknown origin) | J Med Chem 58: 5408-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00658 BindingDB Entry DOI: 10.7270/Q2HX1FDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50152290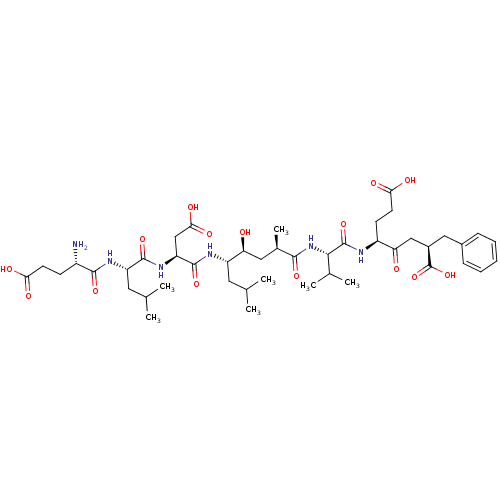 ((2R,5S)-5-[(S)-2-((2R,4S,5S)-5-{(S)-2-[(S)-2-((S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against Beta-secretase | Bioorg Med Chem Lett 14: 4843-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.044 BindingDB Entry DOI: 10.7270/Q2M32WJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50128034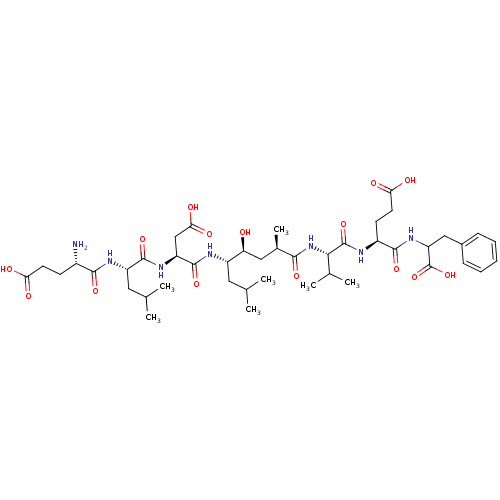 (4-[2-(5-{2-[2-(2-Amino-4-carboxy-butyrylamino)-4-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for human brain memapsin 2 beta-Secretase (BACE) | J Med Chem 46: 2074-82 (2003) Article DOI: 10.1021/jm020513b BindingDB Entry DOI: 10.7270/Q2SN09P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50505569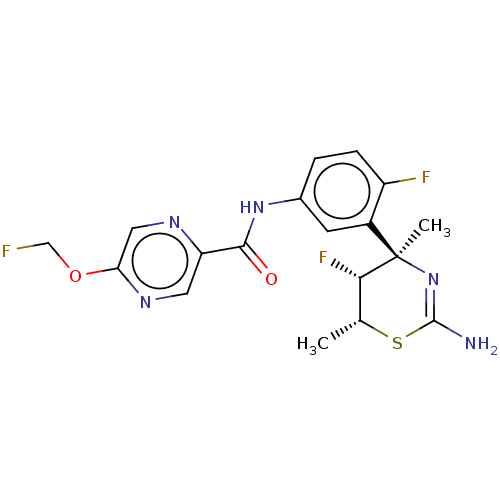 (CHEMBL4557670) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50580216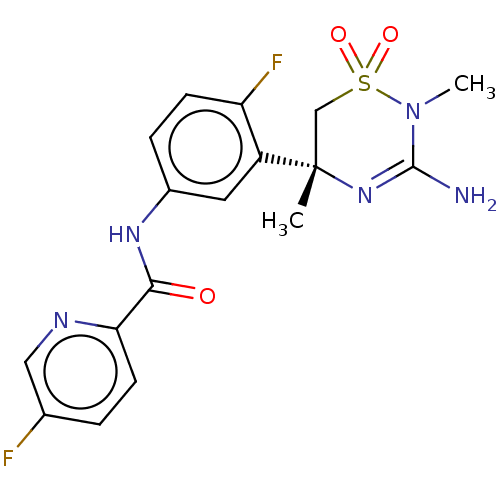 (MK-8931 | SCH 900931 | SCH-900931 | SCH900931 | VE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398473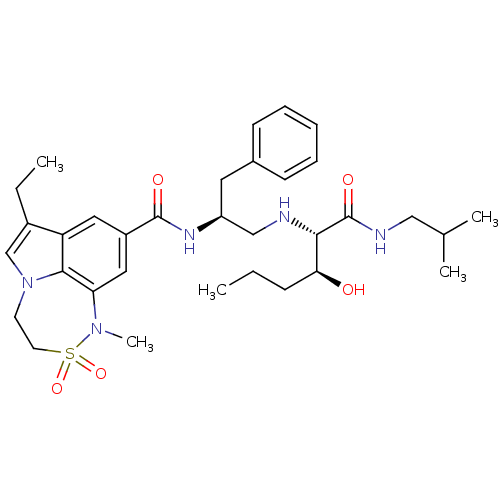 (CHEMBL2179137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398471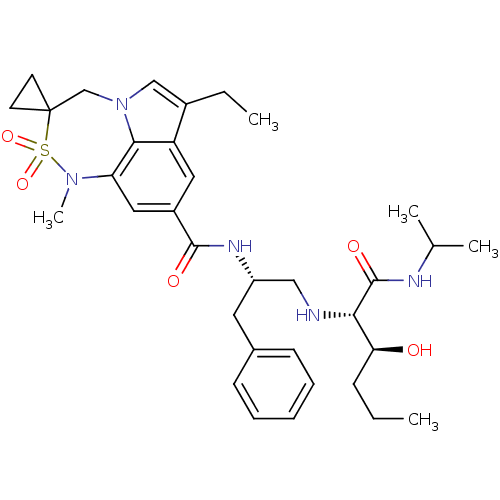 (CHEMBL2179140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli using Arg- Glu(EDANS)-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(Dabcyl)-Arg as substrate by f... | J Med Chem 55: 9195-207 (2012) Article DOI: 10.1021/jm3008823 BindingDB Entry DOI: 10.7270/Q23F4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258901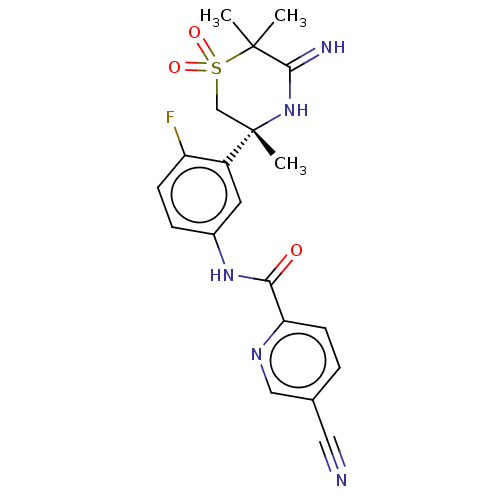 (US9499502, 9b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.5 | <-12.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258899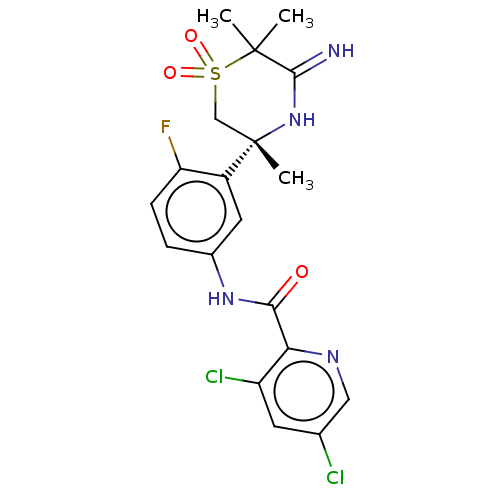 (US9499502, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.5 | <-12.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242250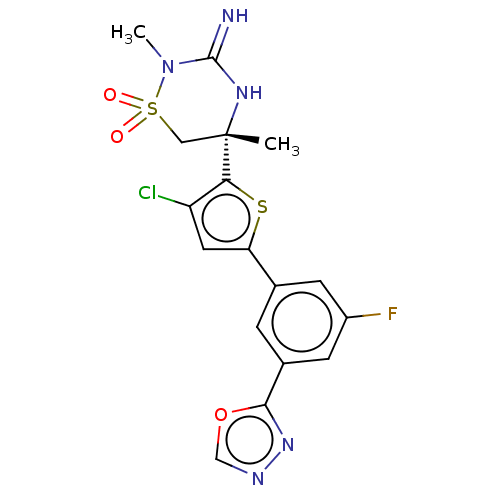 (US9416129, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -12.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258909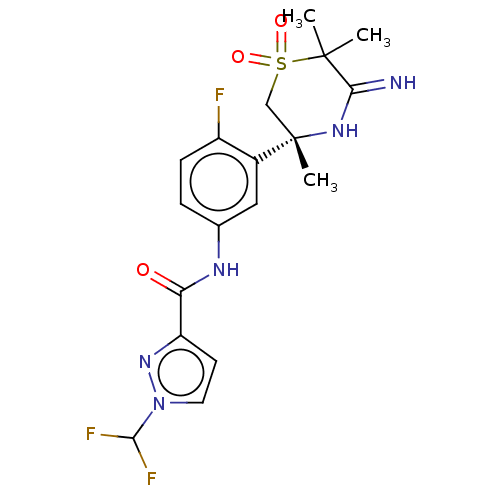 (US9499502, 9j) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -12.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258902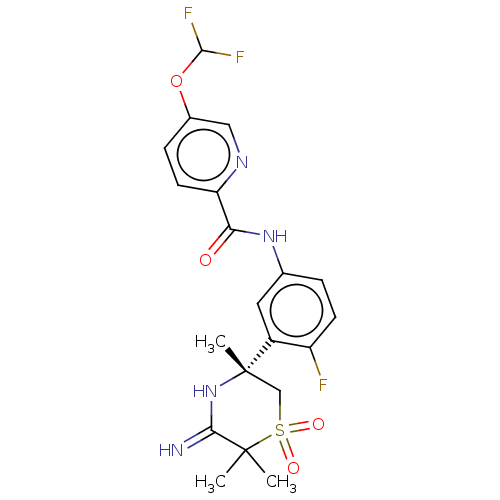 (US9499502, 9c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -12.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258897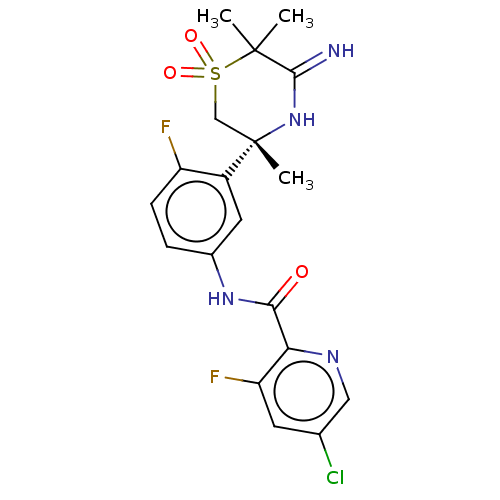 (US9499502, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -12.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335454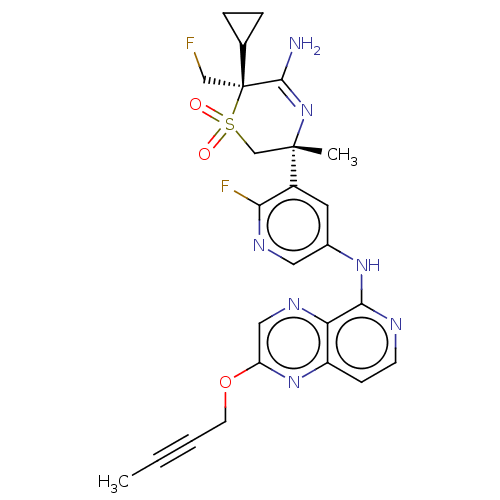 ((3R,6S)-5-amino-3-(5-((2-(but- 2-yn-1-yloxy)pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50580217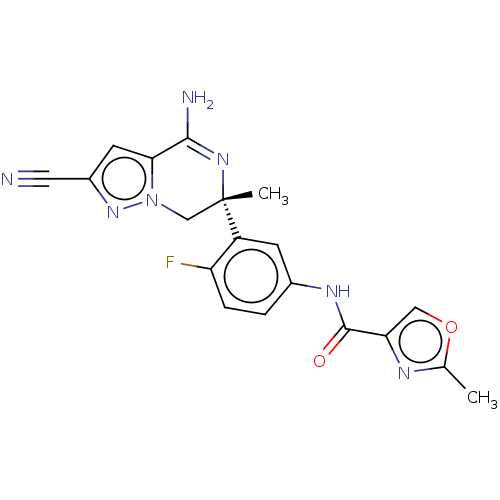 (CHEMBL5093195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to BACE1 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468040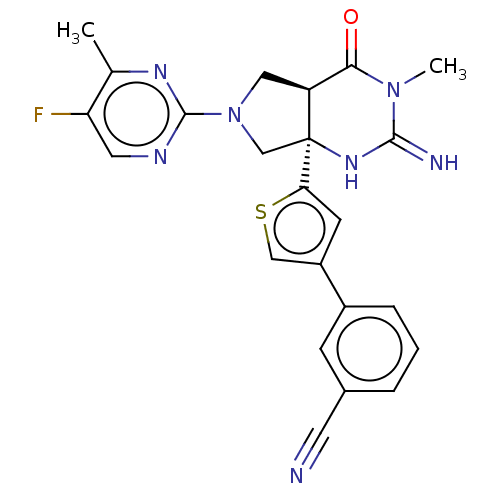 (CHEMBL4289763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258896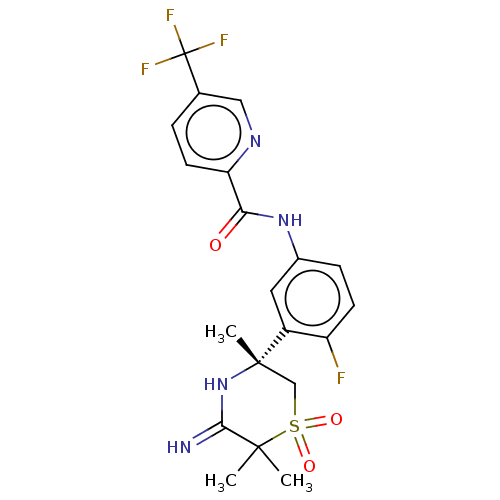 (US9499502, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -12.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258893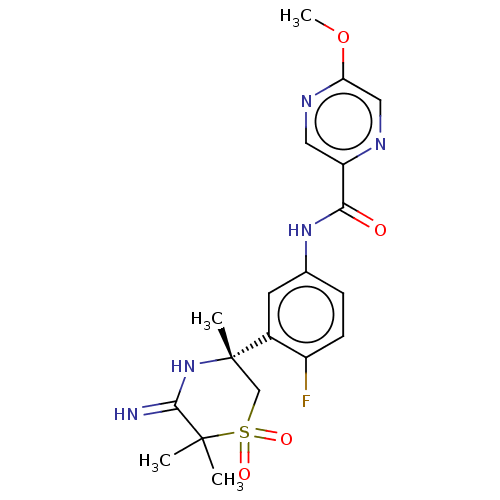 (US9499502, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -12.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258904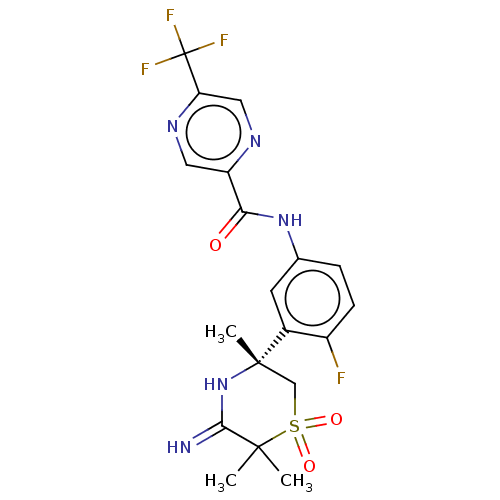 (US9499502, 9e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -12.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468037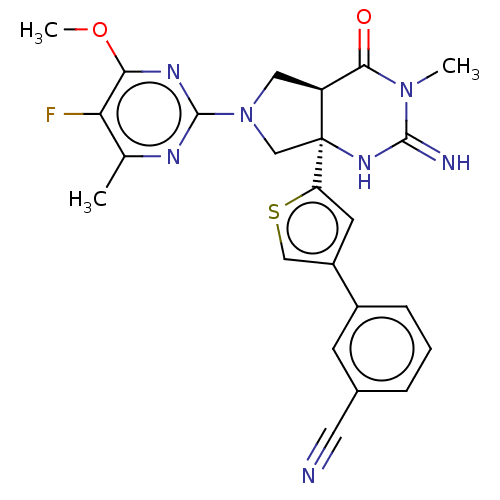 (CHEMBL4293298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335447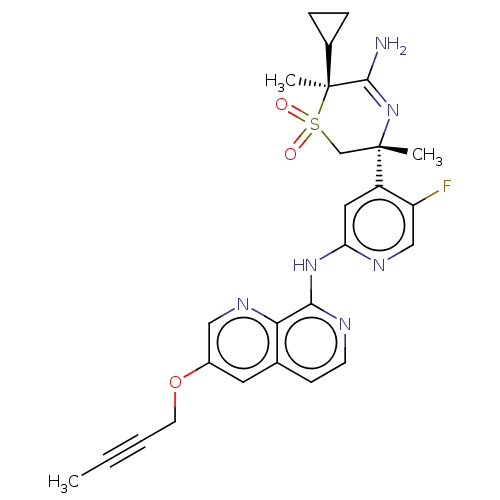 ((3R,6S)-5-amino-3-(2-((3-(but- 2-yn-1-yloxy)-1,7-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267285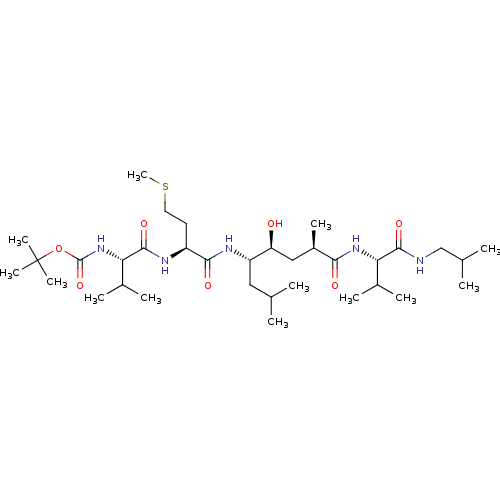 (CHEMBL445804 | tert-butyl (6S,9R,11S,12S,15S,18S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant memapsin 2 | J Med Chem 52: 2163-76 (2009) Article DOI: 10.1021/jm900064c BindingDB Entry DOI: 10.7270/Q2KS6RF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50317048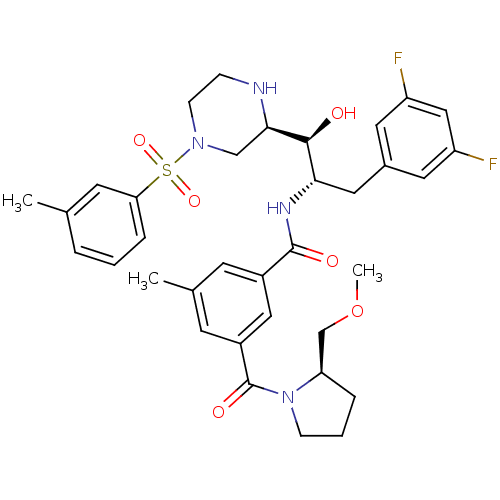 (CHEMBL1097342 | N-((1S,2S)-3-(3,5-difluorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition oh BACE1 | Bioorg Med Chem Lett 20: 2837-42 (2010) Article DOI: 10.1016/j.bmcl.2010.03.050 BindingDB Entry DOI: 10.7270/Q2BK1CH7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579805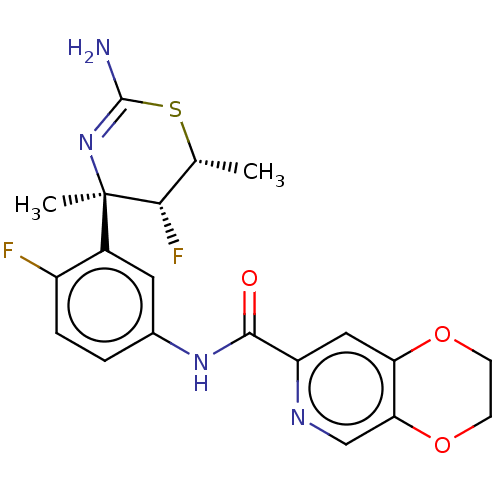 (CHEMBL5075689) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242270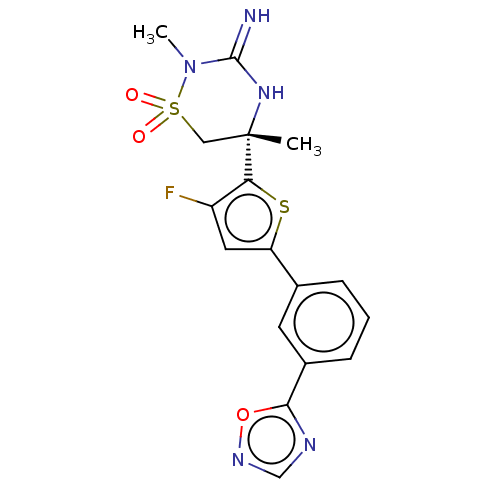 (US9416129, 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.870 | -12.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335466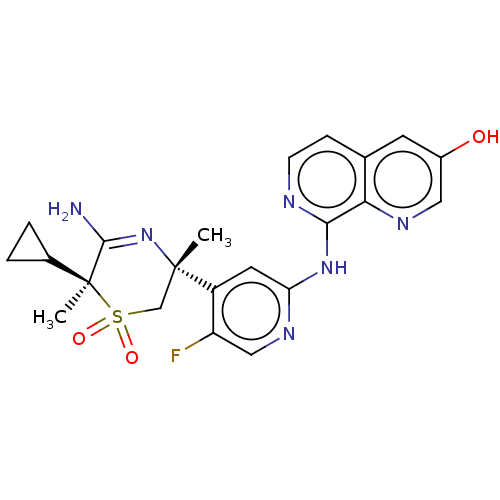 ((3R,6S)-5-amino-6- cyclopropyl-3-(5-fluoro-2-((3- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258892 (US9499502, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM335448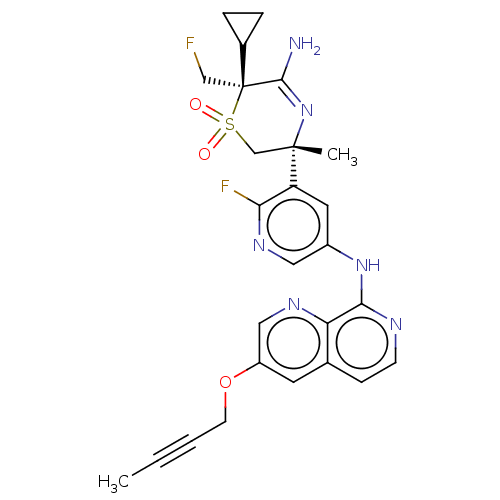 ((3R,6S)-5-amino-3-(5-((3-(but- 2-yn-1-yloxy)-1,7-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention were determined to be potent inhibitors of BACE-1 using the following assay.The following reagents were used in this a... | US Patent US9732088 (2017) BindingDB Entry DOI: 10.7270/Q2XD13S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.949 | n/a | n/a | n/a | n/a | n/a | n/a | 5.01 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The protocol that was used to determine the recited values isdescribed as follows.BACE1 HTRF FRET AssayReagentsNa+-Acetate pH 5.01% Brij-35GlycerolDi... | US Patent US8940748 (2015) BindingDB Entry DOI: 10.7270/Q2ZC81MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.949 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay was used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay monit... | US Patent US9029362 (2015) BindingDB Entry DOI: 10.7270/Q2X63KP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256785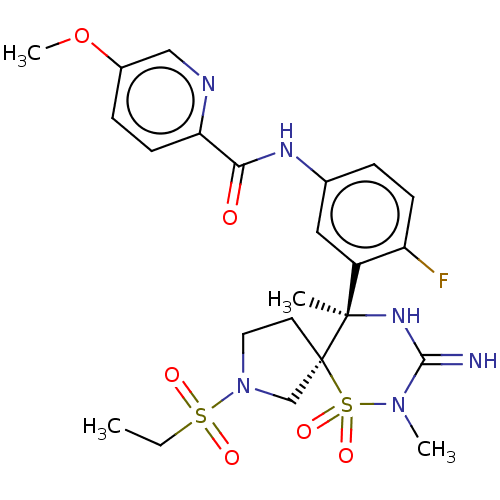 (US9489013, 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256786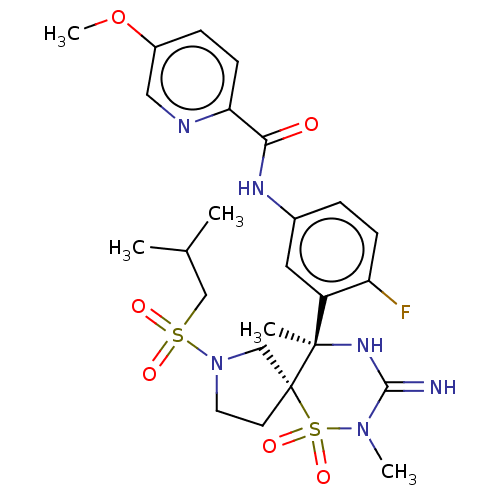 (US9489013, 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256789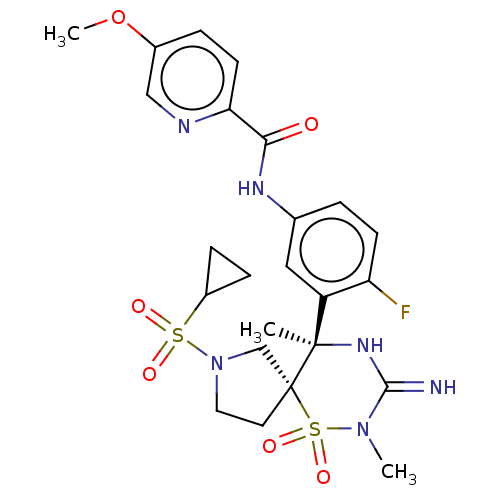 (US9489013, 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256808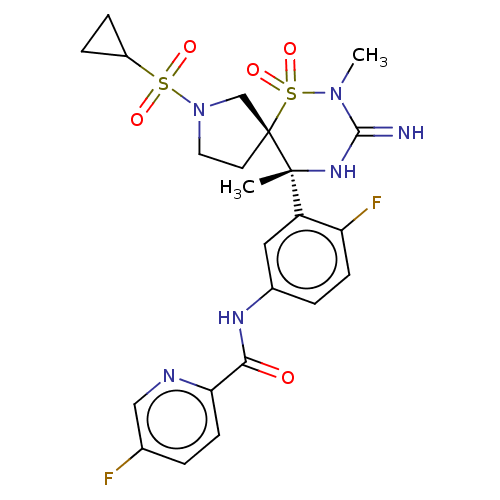 (US9489013, 79) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM256738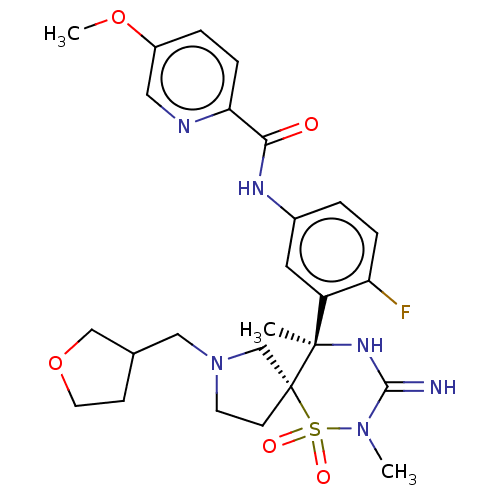 (US9489013, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9489013 (2016) BindingDB Entry DOI: 10.7270/Q2Q81C18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM244046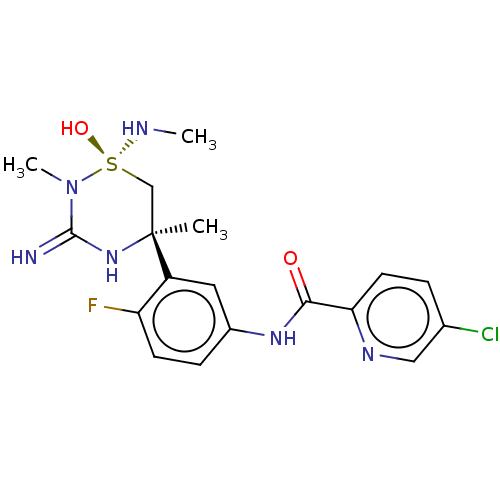 (US9428476, 1b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description This assay monitors the increase of 620 nm fluorescence that resulted from BACE1 cleavage of an APPswedish APPswe mutant peptide FRET substrate (QSY7... | US Patent US9428476 (2016) BindingDB Entry DOI: 10.7270/Q2WQ02Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2581 total ) | Next | Last >> |