

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19518 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19524 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19530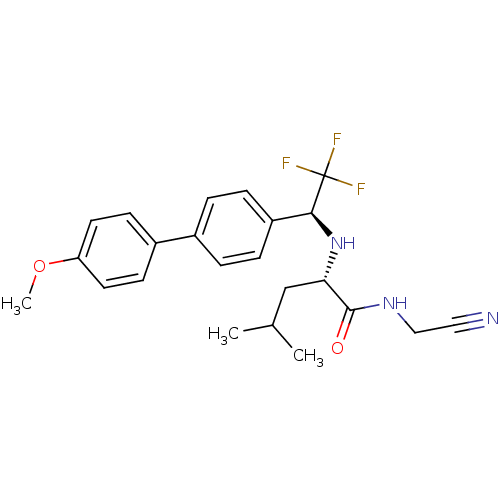 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19489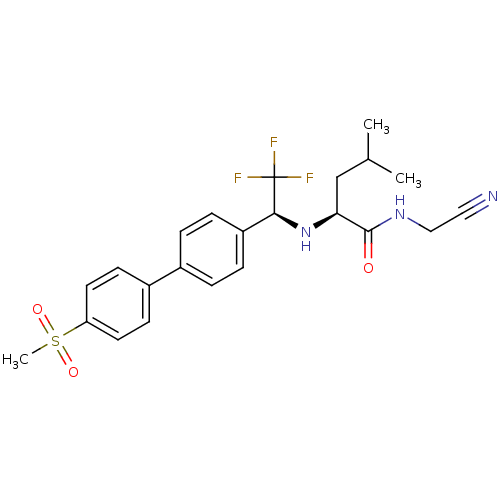 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19533 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19532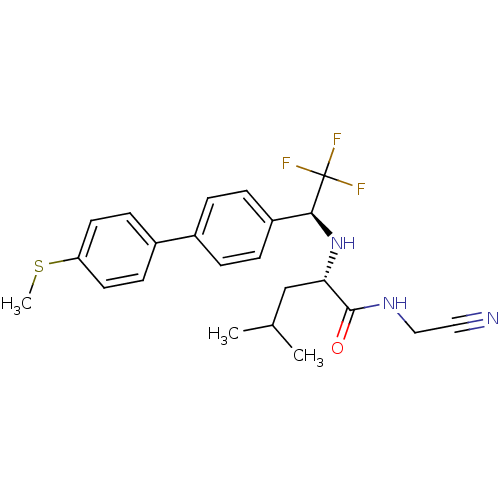 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19538 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19523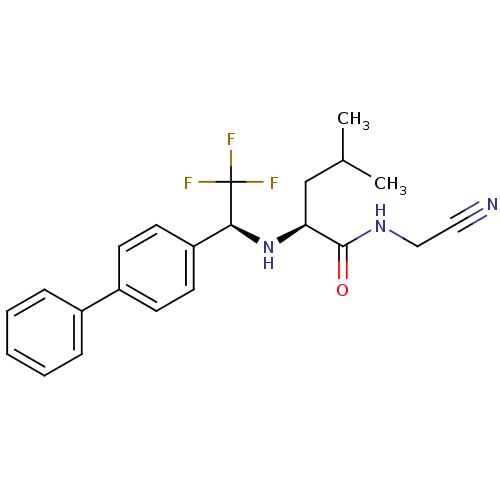 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19544 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19539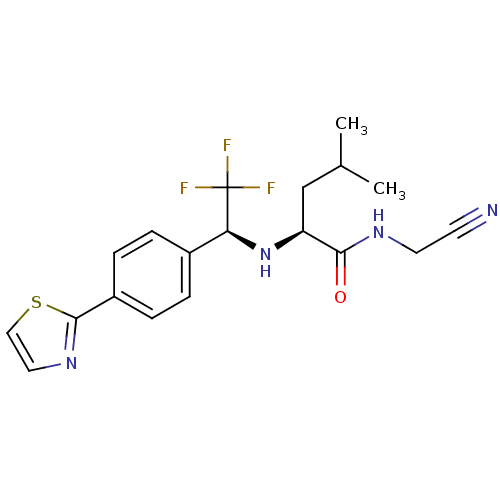 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19534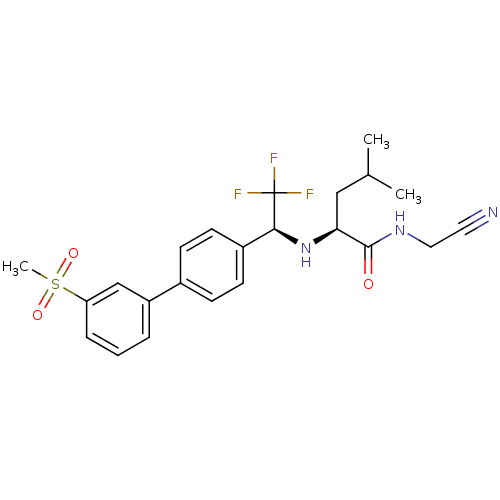 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19529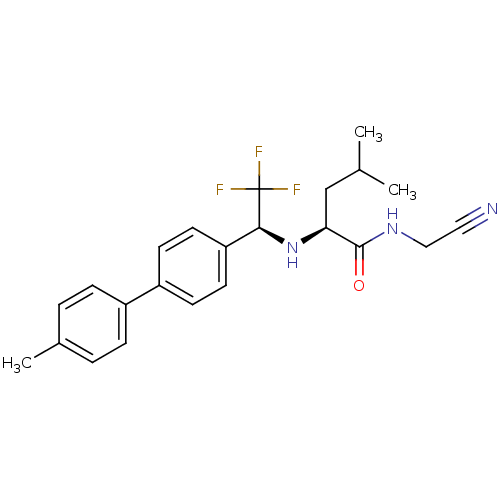 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19537 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19542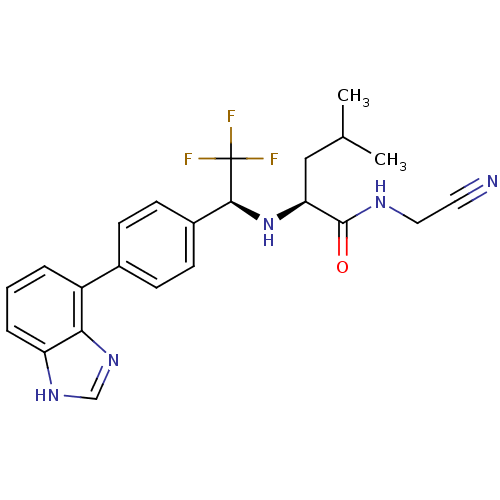 ((2S)-2-{[(1S)-1-[4-(1H-1,3-benzodiazol-4-yl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19525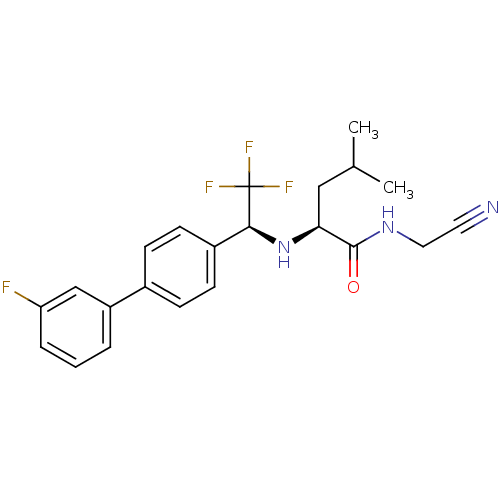 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19526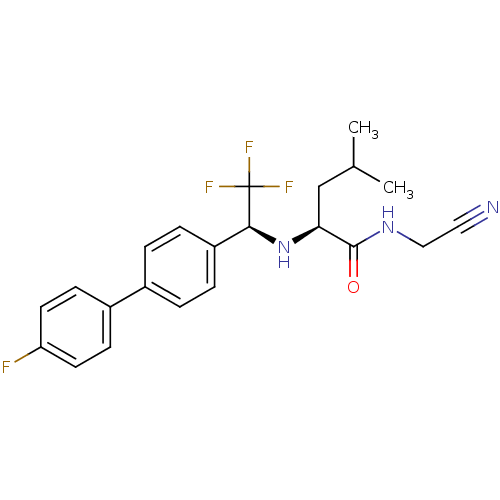 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19522 ((2S)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19531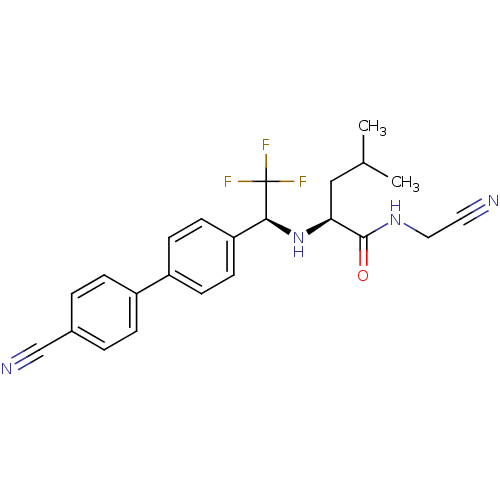 ((2S)-N-(cyanomethyl)-2-{[(1S)-1-[4-(4-cyanophenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19527 ((2S)-N-(cyanomethyl)-2-{[(1S)-1-[4-(3,4-difluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19543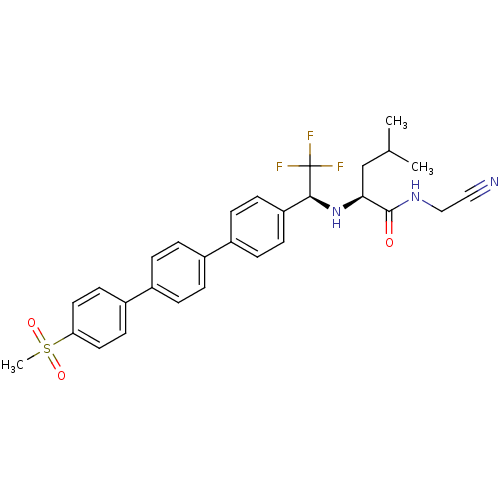 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19536 ((2S)-2-{[(1S)-1-(4-benzoylphenyl)-2,2,2-trifluoroe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19535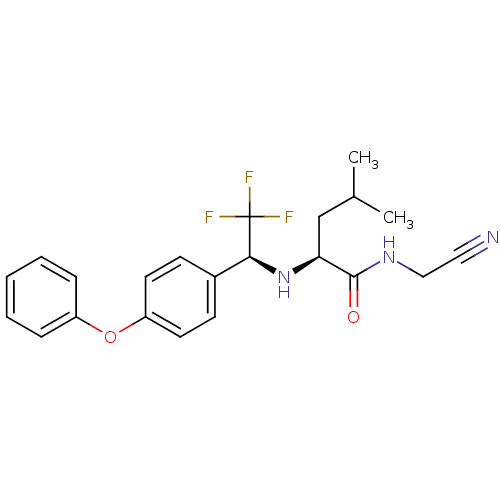 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19540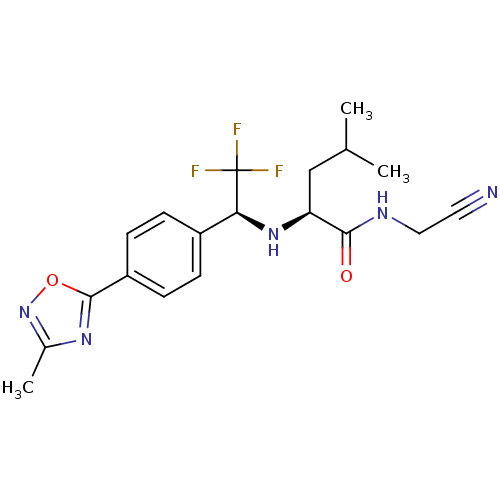 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19528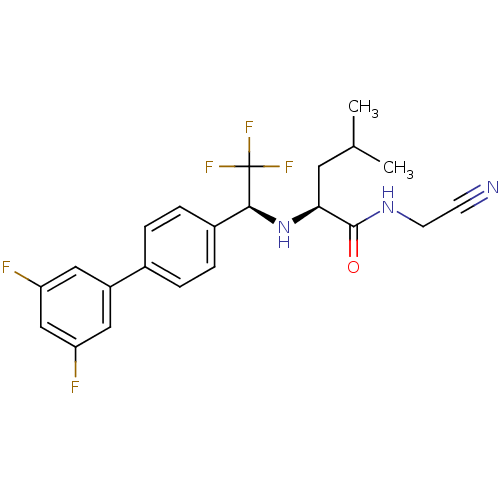 ((2S)-N-(cyanomethyl)-2-{[(1S)-1-[4-(3,5-difluoroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19521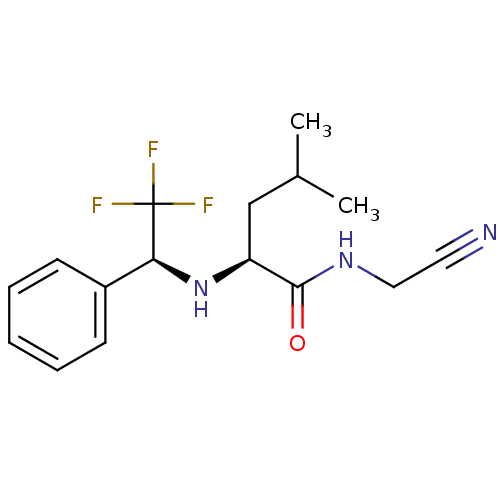 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19522 ((2S)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19521 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19521 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19541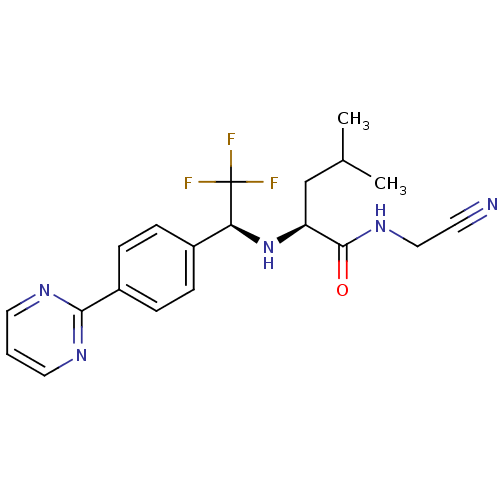 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19518 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19522 ((2S)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19537 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19537 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19532 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19533 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19531 ((2S)-N-(cyanomethyl)-2-{[(1S)-1-[4-(4-cyanophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19544 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19527 ((2S)-N-(cyanomethyl)-2-{[(1S)-1-[4-(3,4-difluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19530 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19535 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19539 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19528 ((2S)-N-(cyanomethyl)-2-{[(1S)-1-[4-(3,5-difluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 249 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19536 ((2S)-2-{[(1S)-1-(4-benzoylphenyl)-2,2,2-trifluoroe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19489 ((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19534 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19525 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19529 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19523 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19540 ((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | Bioorg Med Chem Lett 16: 1985-9 (2006) Article DOI: 10.1016/j.bmcl.2005.12.071 BindingDB Entry DOI: 10.7270/Q237770H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |