

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20132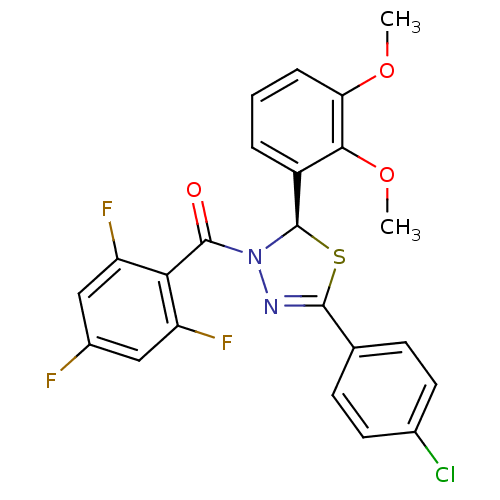 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20132 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20136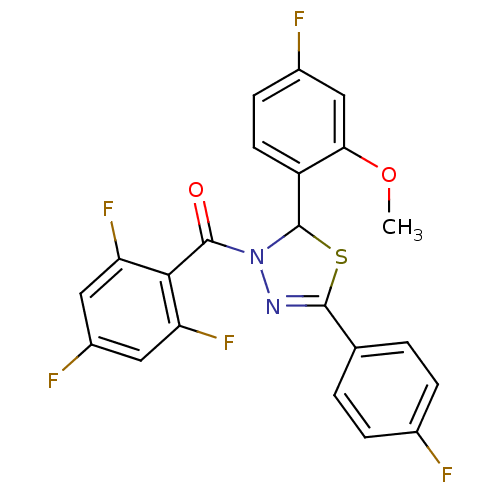 (2-(4-fluoro-2-methoxyphenyl)-5-(4-fluorophenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20137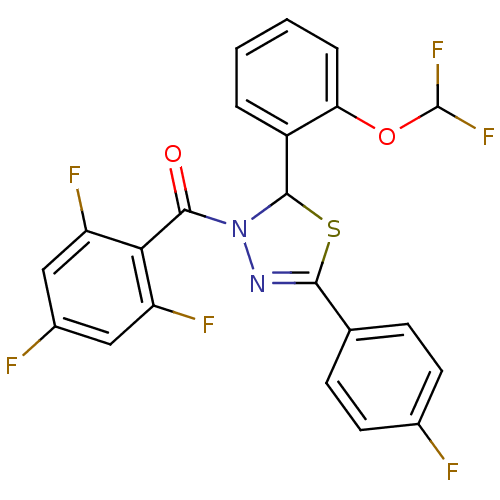 (2-[2-(difluoromethoxy)phenyl]-5-(4-fluorophenyl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20138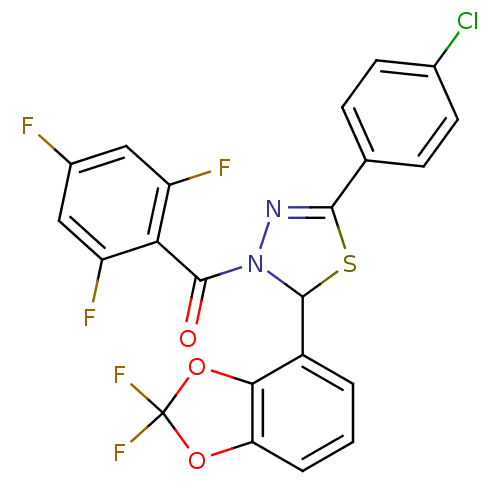 (5-(4-chlorophenyl)-2-(2,2-difluoro-2H-1,3-benzodio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20139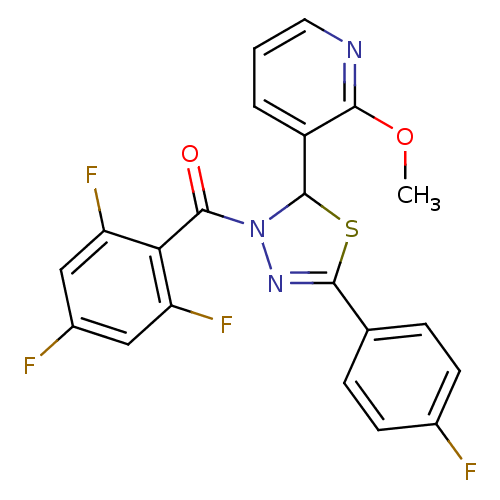 (5-(4-fluorophenyl)-2-(2-methoxypyridin-3-yl)-3-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20140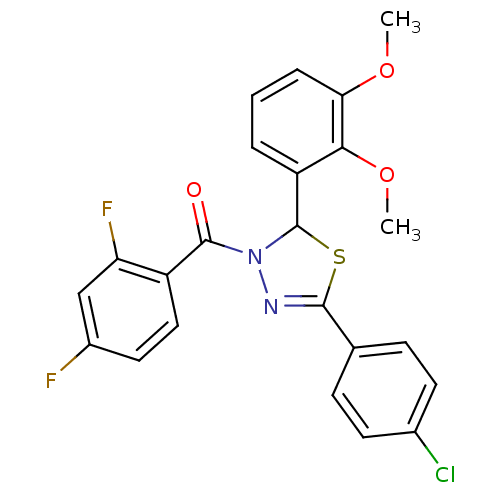 (5-(4-chlorophenyl)-3-[(2,4-difluorophenyl)carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20141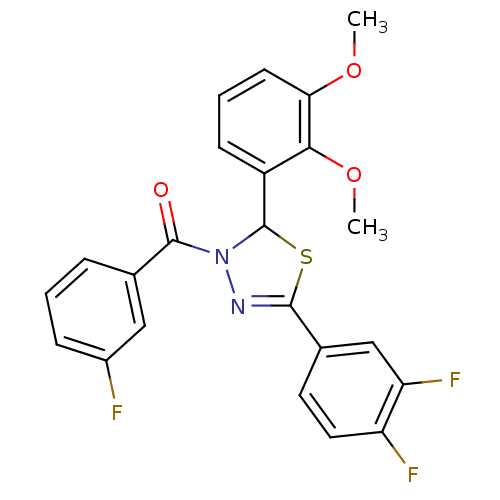 (5-(3,4-difluorophenyl)-2-(2,3-dimethoxyphenyl)-3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20142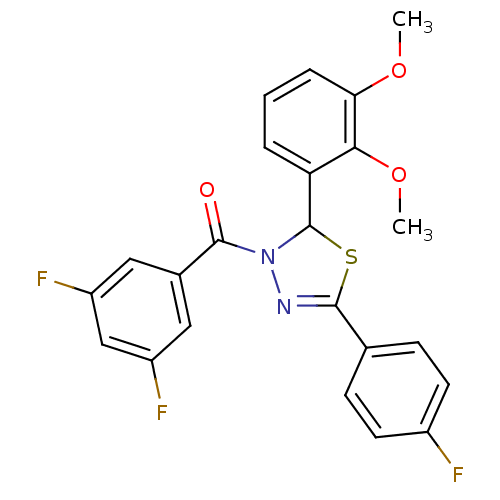 (3-[(3,5-difluorophenyl)carbonyl]-2-(2,3-dimethoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20143 (2-(2,3-dimethoxyphenyl)-3-[(5-fluoro-2-methylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20144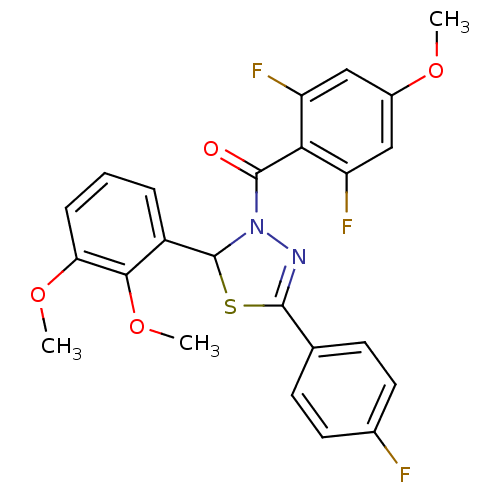 (3-[(2,6-difluoro-4-methoxyphenyl)carbonyl]-2-(2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20145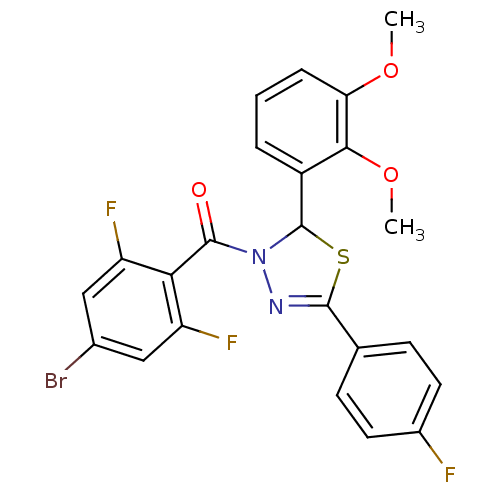 (3-[(4-bromo-2,6-difluorophenyl)carbonyl]-2-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20146 (2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20132 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 67 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20133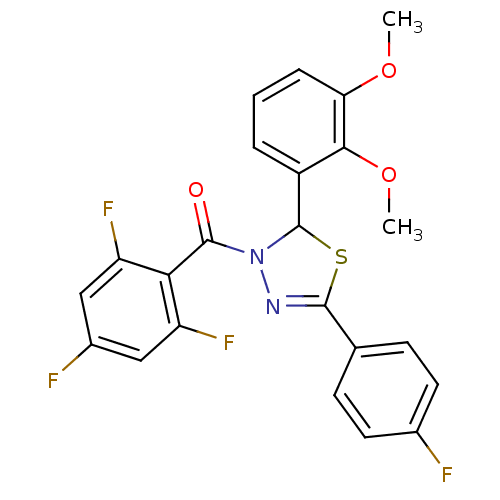 (2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 76 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20134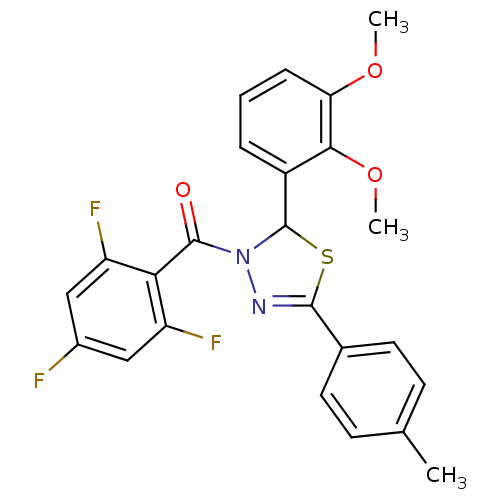 (2-(2,3-dimethoxyphenyl)-5-(4-methylphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20147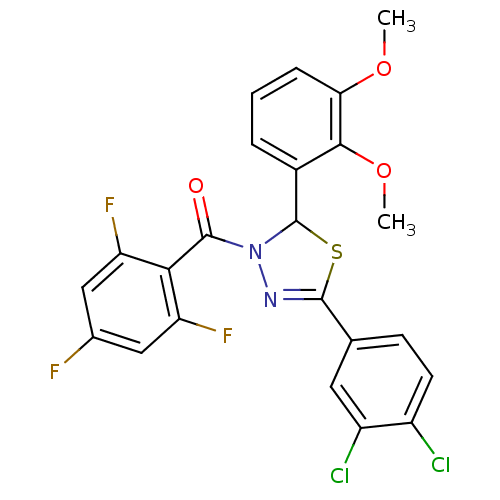 (5-(3,4-dichlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20135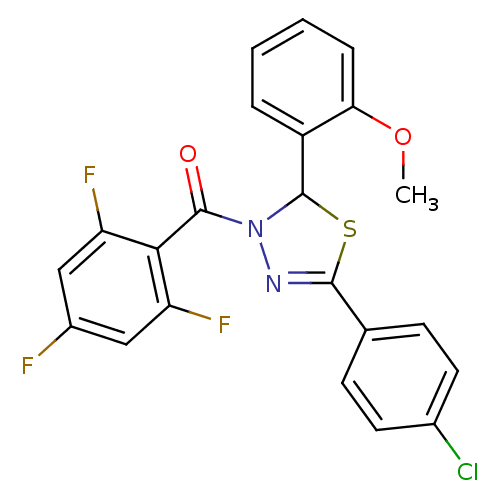 (5-(4-chlorophenyl)-2-(2-methoxyphenyl)-3-[(2,4,6-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20148 (5-(4-chlorophenyl)-2-(3-methoxyphenyl)-3-[(2,4,6-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20136 (2-(4-fluoro-2-methoxyphenyl)-5-(4-fluorophenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20149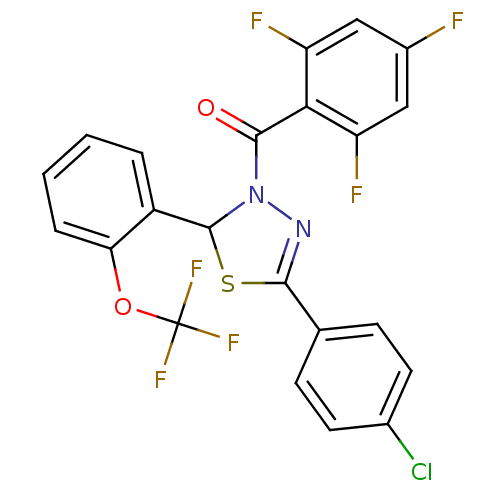 (5-(4-chlorophenyl)-2-[2-(trifluoromethoxy)phenyl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20137 (2-[2-(difluoromethoxy)phenyl]-5-(4-fluorophenyl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20138 (5-(4-chlorophenyl)-2-(2,2-difluoro-2H-1,3-benzodio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20139 (5-(4-fluorophenyl)-2-(2-methoxypyridin-3-yl)-3-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20150 (5-(4-fluorophenyl)-2-(2-methoxyphenyl)-2-methyl-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20151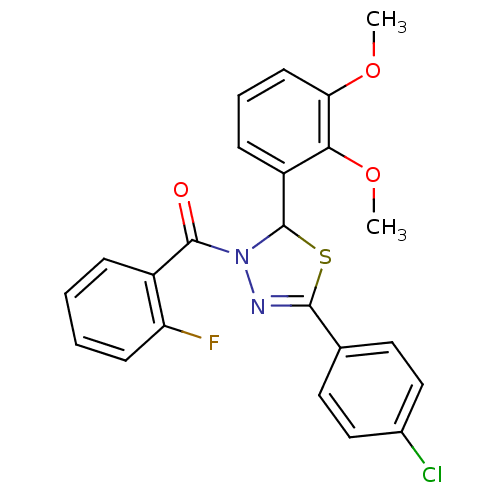 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20140 (5-(4-chlorophenyl)-3-[(2,4-difluorophenyl)carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 980 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20152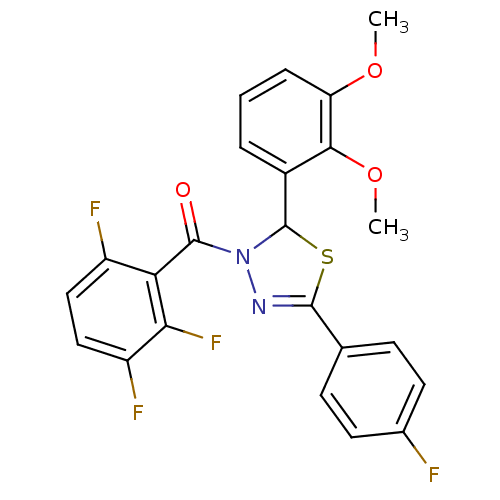 (2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20153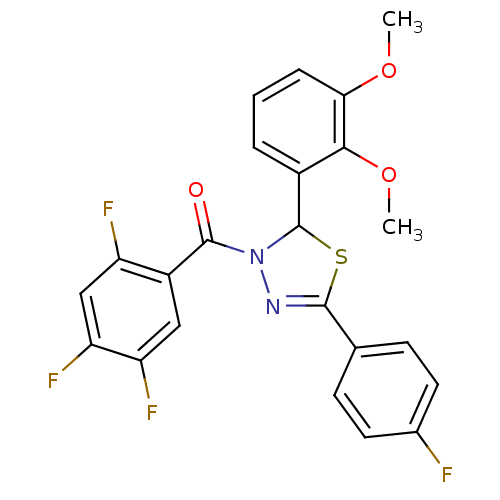 (2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20141 (5-(3,4-difluorophenyl)-2-(2,3-dimethoxyphenyl)-3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20142 (3-[(3,5-difluorophenyl)carbonyl]-2-(2,3-dimethoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20154 (3-[(3-chlorophenyl)carbonyl]-2-(2,3-dimethoxypheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20143 (2-(2,3-dimethoxyphenyl)-3-[(5-fluoro-2-methylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20144 (3-[(2,6-difluoro-4-methoxyphenyl)carbonyl]-2-(2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20145 (3-[(4-bromo-2,6-difluorophenyl)carbonyl]-2-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20146 (2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20155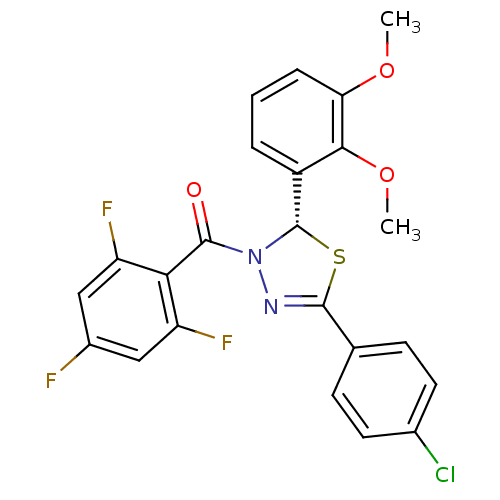 ((2S)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20155 ((2S)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20155 ((2S)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20135 (5-(4-chlorophenyl)-2-(2-methoxyphenyl)-3-[(2,4,6-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20134 (2-(2,3-dimethoxyphenyl)-5-(4-methylphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20133 (2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20132 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20155 ((2S)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |