

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238347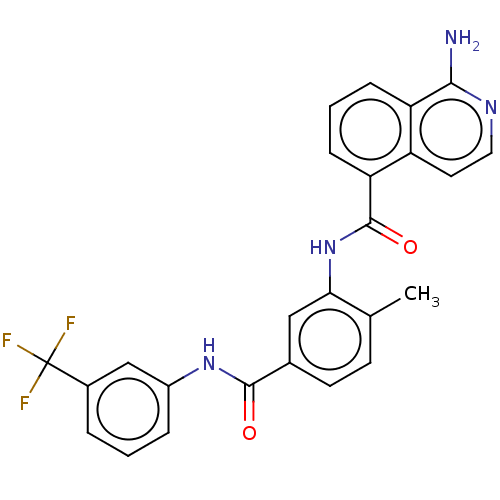 (US9388165, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238345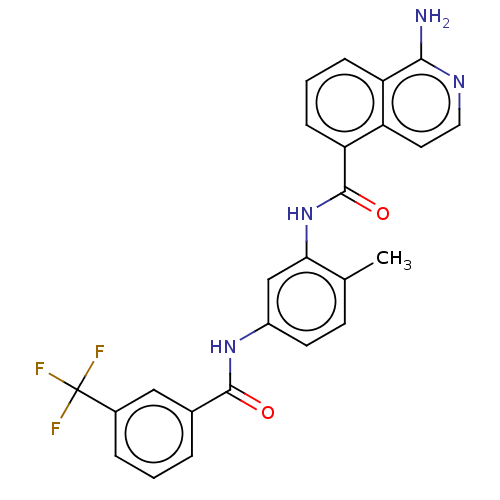 (US9388165, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238349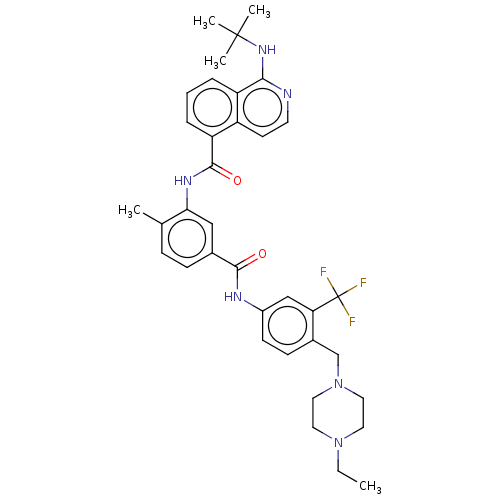 (US9388165, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238348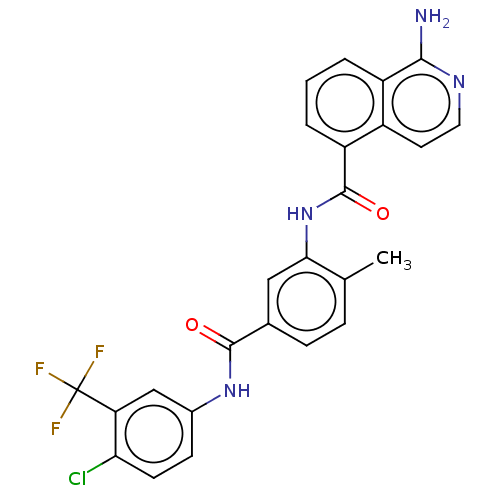 (US9388165, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238346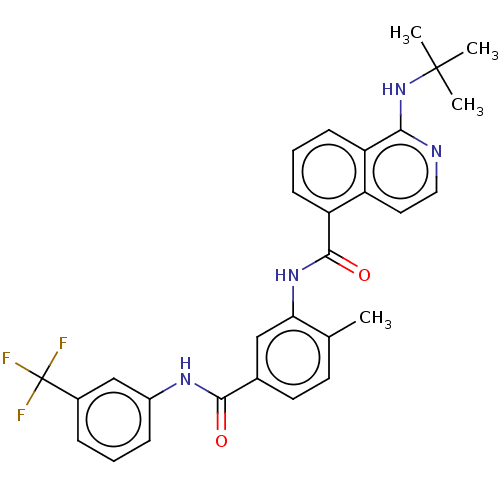 (US9388165, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238350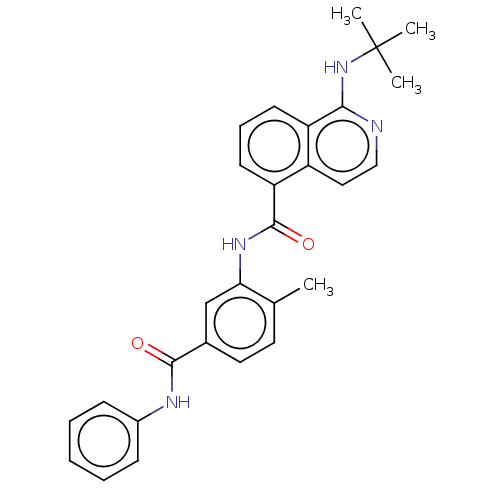 (US9388165, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238351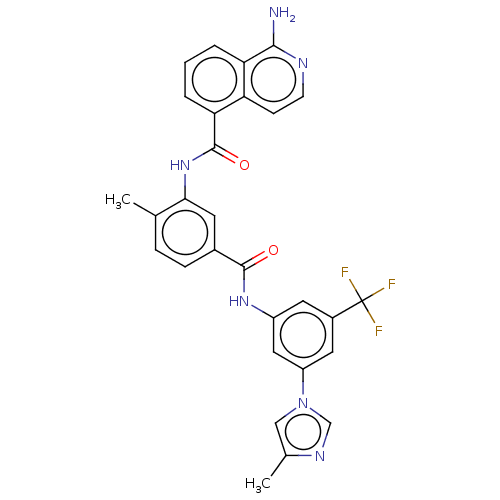 (US9388165, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 94.8 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM238352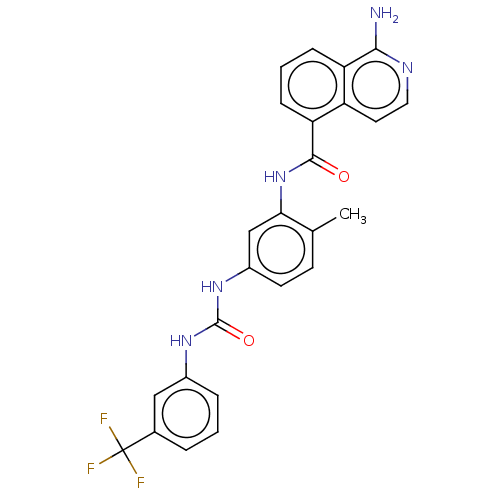 (US9388165, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase [Y340D,Y341D] (Homo sapiens (Human)) | BDBM50396483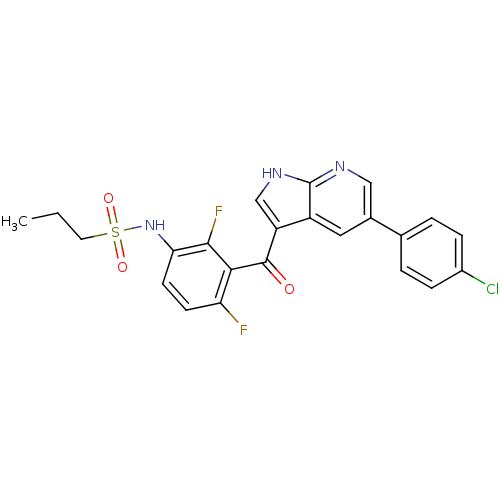 (PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||