

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016767 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016767 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit neutral endopeptidase purified from rabbit kidney | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016766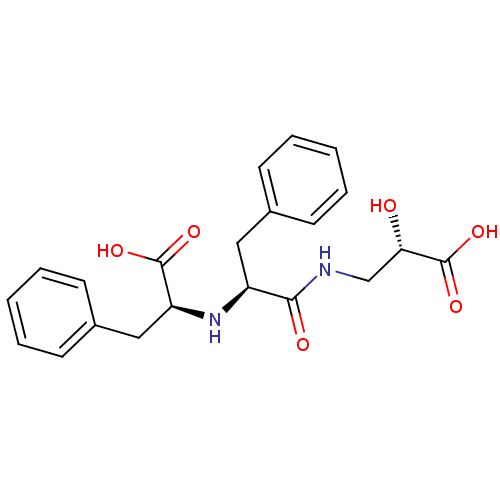 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit neutral endopeptidase purified from rabbit kidney | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016769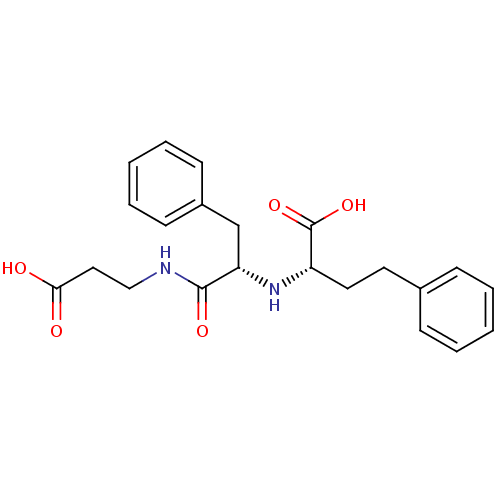 (2-[1-(2-Carboxy-ethylcarbamoyl)-2-phenyl-ethylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit neutral endopeptidase purified from rabbit kidney | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016765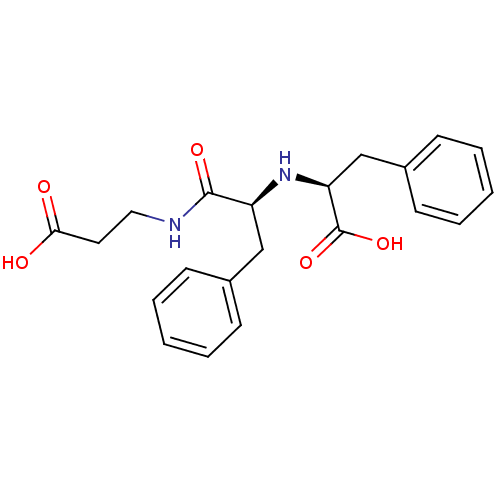 (2-[1-(2-Carboxy-ethylcarbamoyl)-2-phenyl-ethylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit neutral endopeptidase purified from rabbit kidney | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016768 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit neutral endopeptidase purified from rabbit kidney | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016764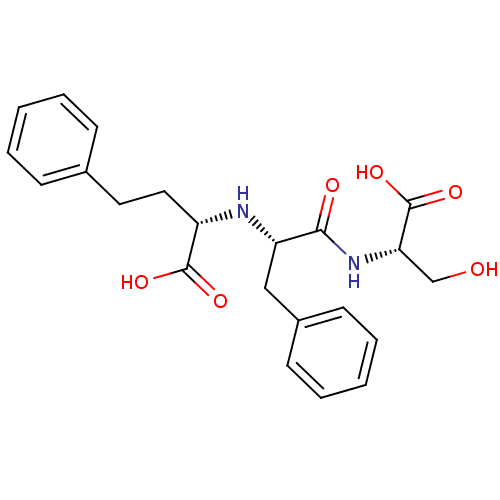 (2-[1-(1-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50016764 (2-[1-(1-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit neutral endopeptidase purified from rabbit kidney | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016768 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016769 (2-[1-(2-Carboxy-ethylcarbamoyl)-2-phenyl-ethylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016767 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016767 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016766 (2-[1-(2-Carboxy-2-hydroxy-ethylcarbamoyl)-2-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50016765 (2-[1-(2-Carboxy-ethylcarbamoyl)-2-phenyl-ethylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Angiotensin I converting enzyme in rat | J Med Chem 32: 737-9 (1989) BindingDB Entry DOI: 10.7270/Q2BV7FMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||