

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50137352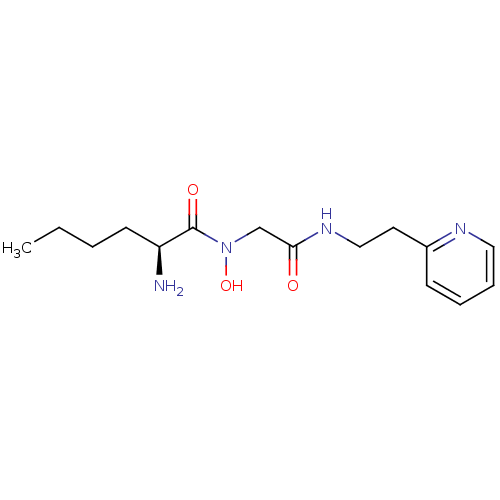 ((S)-2-Amino-hexanoic acid hydroxy-[(2-pyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 1 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50137355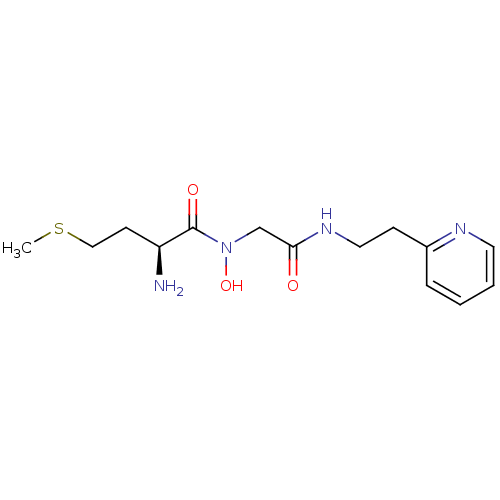 ((S)-2-Amino-N-hydroxy-4-methylsulfanyl-N-[(2-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 1 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137355 ((S)-2-Amino-N-hydroxy-4-methylsulfanyl-N-[(2-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137352 ((S)-2-Amino-hexanoic acid hydroxy-[(2-pyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50137354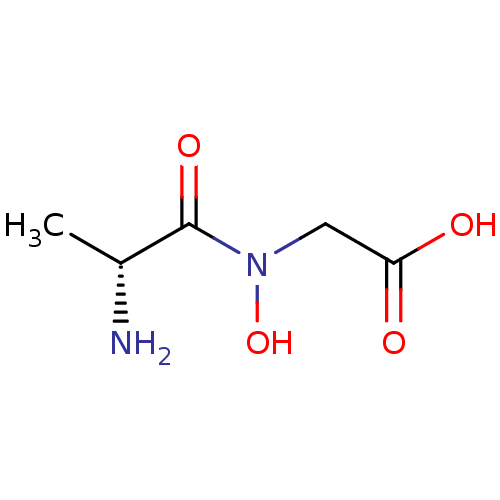 (CHEMBL164406 | [((R)-2-Amino-propionyl)-hydroxy-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 1 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50137353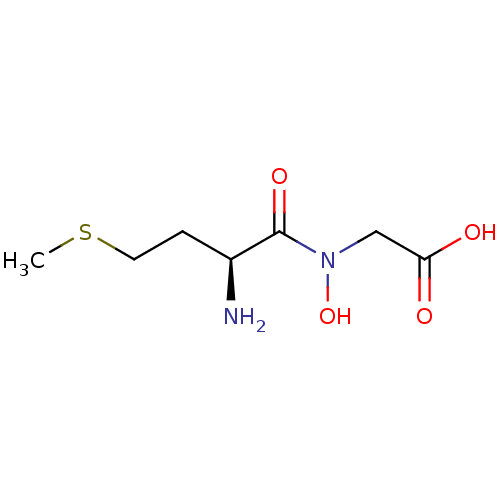 (CHEMBL349154 | [((S)-2-Amino-4-methylsulfanyl-buty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 1 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137354 (CHEMBL164406 | [((R)-2-Amino-propionyl)-hydroxy-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50137353 (CHEMBL349154 | [((S)-2-Amino-4-methylsulfanyl-buty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Methionine aminopeptidase 2 | Bioorg Med Chem Lett 14: 77-9 (2003) BindingDB Entry DOI: 10.7270/Q2DV1J90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||