 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50017009
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50017009 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176375
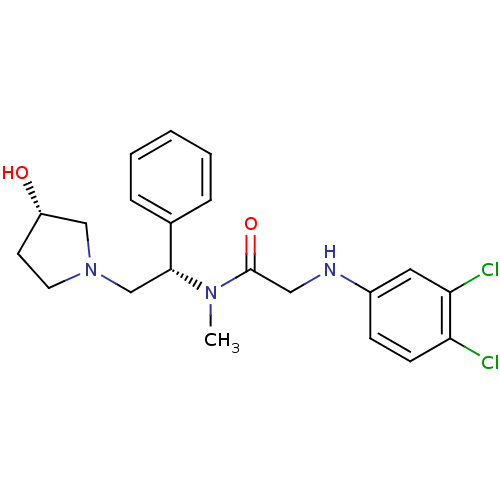
(2-(3,4-dichlorophenylamino)-N-((S)-2-((S)-3-hydrox...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c1-25(21(28)12-24-16-7-8-18(22)19(23)11-16)20(15-5-3-2-4-6-15)14-26-10-9-17(27)13-26/h2-8,11,17,20,24,27H,9-10,12-14H2,1H3/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176369
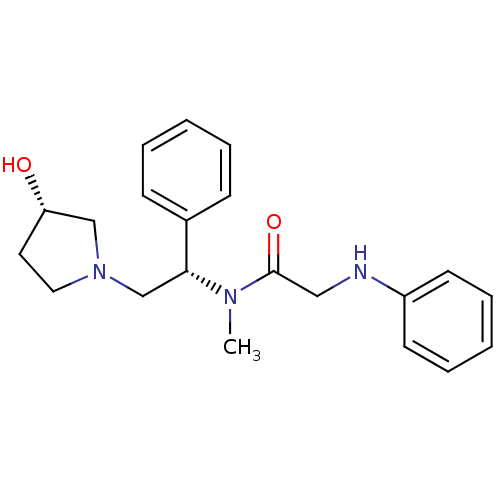
(CHEMBL201884 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1 Show InChI InChI=1S/C21H27N3O2/c1-23(21(26)14-22-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-24-13-12-19(25)15-24/h2-11,19-20,22,25H,12-16H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176370
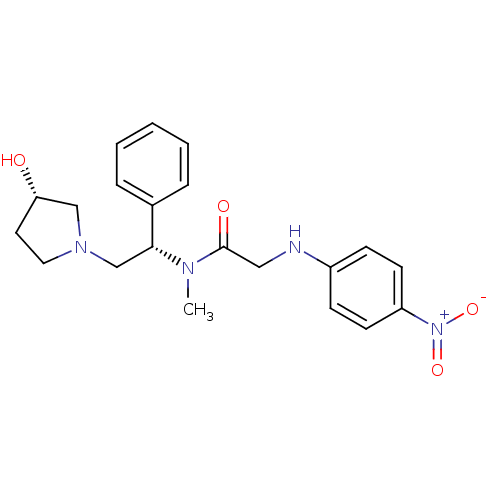
(CHEMBL201572 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-23(21(27)13-22-17-7-9-18(10-8-17)25(28)29)20(16-5-3-2-4-6-16)15-24-12-11-19(26)14-24/h2-10,19-20,22,26H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176374

(CHEMBL382932 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H29N3O2/c1-23(19-11-7-4-8-12-19)17-22(27)24(2)21(18-9-5-3-6-10-18)16-25-14-13-20(26)15-25/h3-12,20-21,26H,13-17H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176365
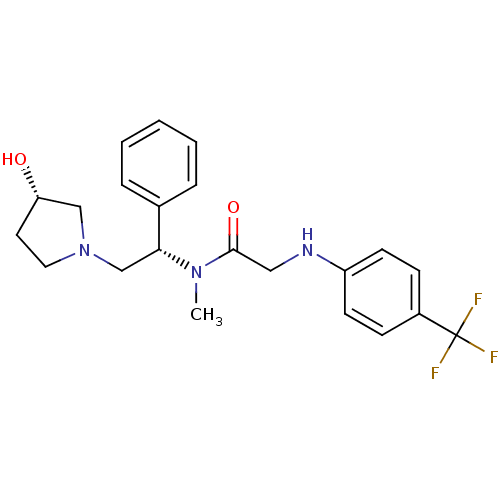
(CHEMBL201905 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c1-27(21(30)13-26-18-9-7-17(8-10-18)22(23,24)25)20(16-5-3-2-4-6-16)15-28-12-11-19(29)14-28/h2-10,19-20,26,29H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176373
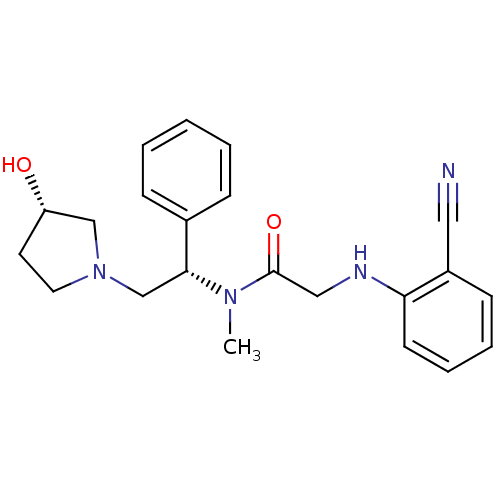
(2-(2-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-20-10-6-5-9-18(20)13-23)21(17-7-3-2-4-8-17)16-26-12-11-19(27)15-26/h2-10,19,21,24,27H,11-12,14-16H2,1H3/t19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176367
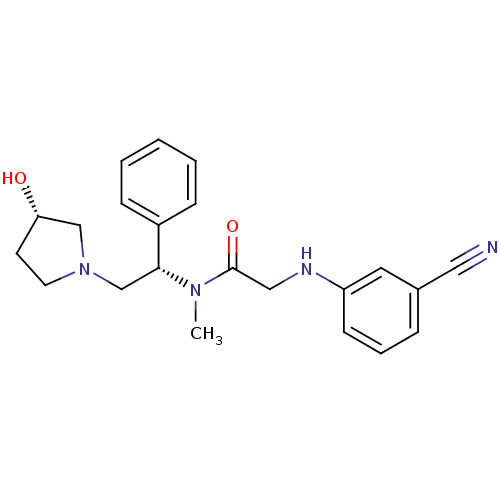
(2-(3-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(c1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-5-6-17(12-19)13-23)21(18-7-3-2-4-8-18)16-26-11-10-20(27)15-26/h2-9,12,20-21,24,27H,10-11,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176376
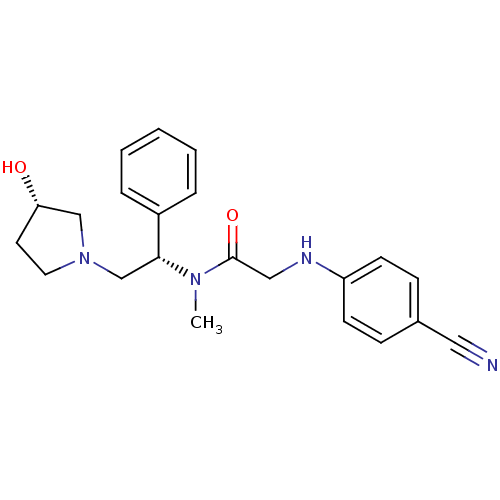
(2-(4-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-7-17(13-23)8-10-19)21(18-5-3-2-4-6-18)16-26-12-11-20(27)15-26/h2-10,20-21,24,27H,11-12,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176372
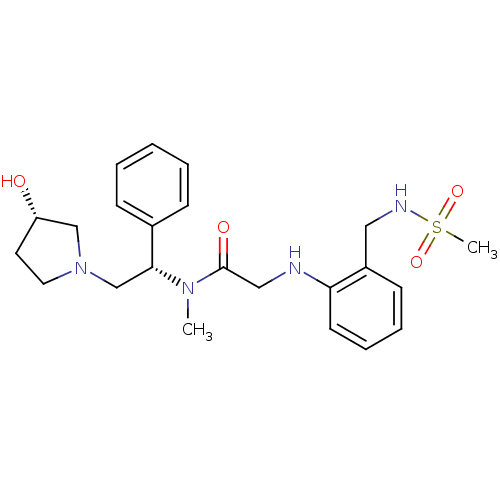
(CHEMBL201283 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1CNS(C)(=O)=O Show InChI InChI=1S/C23H32N4O4S/c1-26(22(18-8-4-3-5-9-18)17-27-13-12-20(28)16-27)23(29)15-24-21-11-7-6-10-19(21)14-25-32(2,30)31/h3-11,20,22,24-25,28H,12-17H2,1-2H3/t20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176362

(2-((4-cyanophenyl)(methyl)amino)-N-((S)-2-((S)-3-h...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H28N4O2/c1-25(20-10-8-18(14-24)9-11-20)17-23(29)26(2)22(19-6-4-3-5-7-19)16-27-13-12-21(28)15-27/h3-11,21-22,28H,12-13,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176371

(CHEMBL202267 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CCCS(=O)(=O)Nc1ccc(NCC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C24H34N4O4S/c1-3-15-33(31,32)26-21-11-9-20(10-12-21)25-16-24(30)27(2)23(19-7-5-4-6-8-19)18-28-14-13-22(29)17-28/h4-12,22-23,25-26,29H,3,13-18H2,1-2H3/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176364

(2-(Acetyl-phenyl-amino)-N-[(S)-2-((S)-3-hydroxy-py...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CN(C(C)=O)c1ccccc1 Show InChI InChI=1S/C23H29N3O3/c1-18(27)26(20-11-7-4-8-12-20)17-23(29)24(2)22(19-9-5-3-6-10-19)16-25-14-13-21(28)15-25/h3-12,21-22,28H,13-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176366
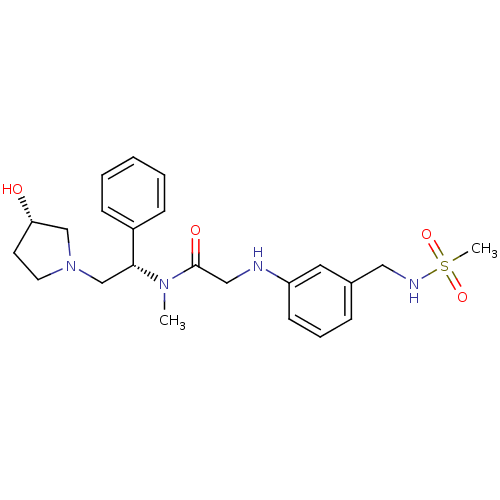
(CHEMBL204650 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(CNS(C)(=O)=O)c1 Show InChI InChI=1S/C23H32N4O4S/c1-26(22(19-8-4-3-5-9-19)17-27-12-11-21(28)16-27)23(29)15-24-20-10-6-7-18(13-20)14-25-32(2,30)31/h3-10,13,21-22,24-25,28H,11-12,14-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176363
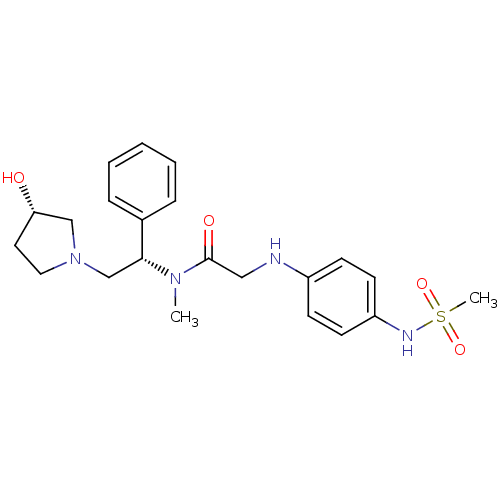
(CHEMBL369993 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H30N4O4S/c1-25(21(17-6-4-3-5-7-17)16-26-13-12-20(27)15-26)22(28)14-23-18-8-10-19(11-9-18)24-31(2,29)30/h3-11,20-21,23-24,27H,12-16H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176368

(CHEMBL441124 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(CNS(C)(=O)=O)cc1 Show InChI InChI=1S/C23H32N4O4S/c1-26(22(19-6-4-3-5-7-19)17-27-13-12-21(28)16-27)23(29)15-24-20-10-8-18(9-11-20)14-25-32(2,30)31/h3-11,21-22,24-25,28H,12-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50176364

(2-(Acetyl-phenyl-amino)-N-[(S)-2-((S)-3-hydroxy-py...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CN(C(C)=O)c1ccccc1 Show InChI InChI=1S/C23H29N3O3/c1-18(27)26(20-11-7-4-8-12-20)17-23(29)24(2)22(19-9-5-3-6-10-19)16-25-14-13-21(28)15-25/h3-12,21-22,28H,13-17H2,1-2H3/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50176363

(CHEMBL369993 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H30N4O4S/c1-25(21(17-6-4-3-5-7-17)16-26-13-12-20(27)15-26)22(28)14-23-18-8-10-19(11-9-18)24-31(2,29)30/h3-11,20-21,23-24,27H,12-16H2,1-2H3/t20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176365

(CHEMBL201905 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c1-27(21(30)13-26-18-9-7-17(8-10-18)22(23,24)25)20(16-5-3-2-4-6-16)15-28-12-11-19(29)14-28/h2-10,19-20,26,29H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176364

(2-(Acetyl-phenyl-amino)-N-[(S)-2-((S)-3-hydroxy-py...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CN(C(C)=O)c1ccccc1 Show InChI InChI=1S/C23H29N3O3/c1-18(27)26(20-11-7-4-8-12-20)17-23(29)24(2)22(19-9-5-3-6-10-19)16-25-14-13-21(28)15-25/h3-12,21-22,28H,13-17H2,1-2H3/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176373

(2-(2-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-20-10-6-5-9-18(20)13-23)21(17-7-3-2-4-8-17)16-26-12-11-19(27)15-26/h2-10,19,21,24,27H,11-12,14-16H2,1H3/t19-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176374

(CHEMBL382932 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H29N3O2/c1-23(19-11-7-4-8-12-19)17-22(27)24(2)21(18-9-5-3-6-10-18)16-25-14-13-20(26)15-25/h3-12,20-21,26H,13-17H2,1-2H3/t20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176362

(2-((4-cyanophenyl)(methyl)amino)-N-((S)-2-((S)-3-h...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H28N4O2/c1-25(20-10-8-18(14-24)9-11-20)17-23(29)26(2)22(19-6-4-3-5-7-19)16-27-13-12-21(28)15-27/h3-11,21-22,28H,12-13,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176375

(2-(3,4-dichlorophenylamino)-N-((S)-2-((S)-3-hydrox...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c1-25(21(28)12-24-16-7-8-18(22)19(23)11-16)20(15-5-3-2-4-6-15)14-26-10-9-17(27)13-26/h2-8,11,17,20,24,27H,9-10,12-14H2,1H3/t17-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176372

(CHEMBL201283 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1CNS(C)(=O)=O Show InChI InChI=1S/C23H32N4O4S/c1-26(22(18-8-4-3-5-9-18)17-27-13-12-20(28)16-27)23(29)15-24-21-11-7-6-10-19(21)14-25-32(2,30)31/h3-11,20,22,24-25,28H,12-17H2,1-2H3/t20-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176376

(2-(4-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-7-17(13-23)8-10-19)21(18-5-3-2-4-6-18)16-26-12-11-20(27)15-26/h2-10,20-21,24,27H,11-12,14-16H2,1H3/t20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176367

(2-(3-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(c1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-5-6-17(12-19)13-23)21(18-7-3-2-4-8-18)16-26-11-10-20(27)15-26/h2-9,12,20-21,24,27H,10-11,14-16H2,1H3/t20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176370

(CHEMBL201572 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-23(21(27)13-22-17-7-9-18(10-8-17)25(28)29)20(16-5-3-2-4-6-16)15-24-12-11-19(26)14-24/h2-10,19-20,22,26H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176369

(CHEMBL201884 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1 Show InChI InChI=1S/C21H27N3O2/c1-23(21(26)14-22-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-24-13-12-19(25)15-24/h2-11,19-20,22,25H,12-16H2,1H3/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176368

(CHEMBL441124 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(CNS(C)(=O)=O)cc1 Show InChI InChI=1S/C23H32N4O4S/c1-26(22(19-6-4-3-5-7-19)17-27-13-12-21(28)16-27)23(29)15-24-20-10-8-18(9-11-20)14-25-32(2,30)31/h3-11,21-22,24-25,28H,12-17H2,1-2H3/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176366

(CHEMBL204650 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(CNS(C)(=O)=O)c1 Show InChI InChI=1S/C23H32N4O4S/c1-26(22(19-8-4-3-5-9-19)17-27-12-11-21(28)16-27)23(29)15-24-20-10-6-7-18(13-20)14-25-32(2,30)31/h3-10,13,21-22,24-25,28H,11-12,14-17H2,1-2H3/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50176371

(CHEMBL202267 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CCCS(=O)(=O)Nc1ccc(NCC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C24H34N4O4S/c1-3-15-33(31,32)26-21-11-9-20(10-12-21)25-16-24(30)27(2)23(19-7-5-4-6-8-19)18-28-14-13-22(29)17-28/h4-12,22-23,25-26,29H,3,13-18H2,1-2H3/t22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176363

(CHEMBL369993 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H30N4O4S/c1-25(21(17-6-4-3-5-7-17)16-26-13-12-20(27)15-26)22(28)14-23-18-8-10-19(11-9-18)24-31(2,29)30/h3-11,20-21,23-24,27H,12-16H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176370

(CHEMBL201572 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-23(21(27)13-22-17-7-9-18(10-8-17)25(28)29)20(16-5-3-2-4-6-16)15-24-12-11-19(26)14-24/h2-10,19-20,22,26H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176374

(CHEMBL382932 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H29N3O2/c1-23(19-11-7-4-8-12-19)17-22(27)24(2)21(18-9-5-3-6-10-18)16-25-14-13-20(26)15-25/h3-12,20-21,26H,13-17H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176365

(CHEMBL201905 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c1-27(21(30)13-26-18-9-7-17(8-10-18)22(23,24)25)20(16-5-3-2-4-6-16)15-28-12-11-19(29)14-28/h2-10,19-20,26,29H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176371

(CHEMBL202267 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CCCS(=O)(=O)Nc1ccc(NCC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C24H34N4O4S/c1-3-15-33(31,32)26-21-11-9-20(10-12-21)25-16-24(30)27(2)23(19-7-5-4-6-8-19)18-28-14-13-22(29)17-28/h4-12,22-23,25-26,29H,3,13-18H2,1-2H3/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176364

(2-(Acetyl-phenyl-amino)-N-[(S)-2-((S)-3-hydroxy-py...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CN(C(C)=O)c1ccccc1 Show InChI InChI=1S/C23H29N3O3/c1-18(27)26(20-11-7-4-8-12-20)17-23(29)24(2)22(19-9-5-3-6-10-19)16-25-14-13-21(28)15-25/h3-12,21-22,28H,13-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176362

(2-((4-cyanophenyl)(methyl)amino)-N-((S)-2-((S)-3-h...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H28N4O2/c1-25(20-10-8-18(14-24)9-11-20)17-23(29)26(2)22(19-6-4-3-5-7-19)16-27-13-12-21(28)15-27/h3-11,21-22,28H,12-13,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176375

(2-(3,4-dichlorophenylamino)-N-((S)-2-((S)-3-hydrox...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c1-25(21(28)12-24-16-7-8-18(22)19(23)11-16)20(15-5-3-2-4-6-15)14-26-10-9-17(27)13-26/h2-8,11,17,20,24,27H,9-10,12-14H2,1H3/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176376

(2-(4-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-7-17(13-23)8-10-19)21(18-5-3-2-4-6-18)16-26-12-11-20(27)15-26/h2-10,20-21,24,27H,11-12,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176366

(CHEMBL204650 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(CNS(C)(=O)=O)c1 Show InChI InChI=1S/C23H32N4O4S/c1-26(22(19-8-4-3-5-9-19)17-27-12-11-21(28)16-27)23(29)15-24-20-10-6-7-18(13-20)14-25-32(2,30)31/h3-10,13,21-22,24-25,28H,11-12,14-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176368

(CHEMBL441124 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(CNS(C)(=O)=O)cc1 Show InChI InChI=1S/C23H32N4O4S/c1-26(22(19-6-4-3-5-7-19)17-27-13-12-21(28)16-27)23(29)15-24-20-10-8-18(9-11-20)14-25-32(2,30)31/h3-11,21-22,24-25,28H,12-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176369

(CHEMBL201884 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1 Show InChI InChI=1S/C21H27N3O2/c1-23(21(26)14-22-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-24-13-12-19(25)15-24/h2-11,19-20,22,25H,12-16H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176373

(2-(2-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-20-10-6-5-9-18(20)13-23)21(17-7-3-2-4-8-17)16-26-12-11-19(27)15-26/h2-10,19,21,24,27H,11-12,14-16H2,1H3/t19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176367

(2-(3-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(c1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-5-6-17(12-19)13-23)21(18-7-3-2-4-8-18)16-26-11-10-20(27)15-26/h2-9,12,20-21,24,27H,10-11,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176372

(CHEMBL201283 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1CNS(C)(=O)=O Show InChI InChI=1S/C23H32N4O4S/c1-26(22(18-8-4-3-5-9-18)17-27-13-12-20(28)16-27)23(29)15-24-21-11-7-6-10-19(21)14-25-32(2,30)31/h3-11,20,22,24-25,28H,12-17H2,1-2H3/t20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor in a [35S]GTPgammaS functional assay |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data