 Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50022065
Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50022065 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231280
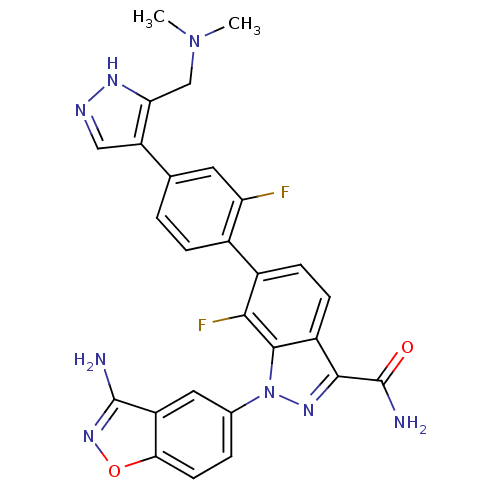
(1-(3-cyano-4-fluorophenyl)-6-[4-(3-dimethylaminome...)Show SMILES CN(C)Cc1[nH]ncc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H22F2N8O2/c1-36(2)12-21-19(11-32-33-21)13-3-5-15(20(28)9-13)16-6-7-17-24(27(31)38)34-37(25(17)23(16)29)14-4-8-22-18(10-14)26(30)35-39-22/h3-11H,12H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231282
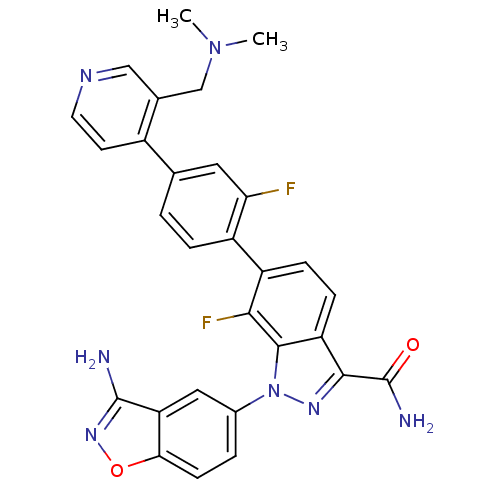
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...)Show SMILES CN(C)Cc1cnccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-13-34-10-9-18(16)15-3-5-19(23(30)11-15)20-6-7-21-26(29(33)39)35-38(27(21)25(20)31)17-4-8-24-22(12-17)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231275
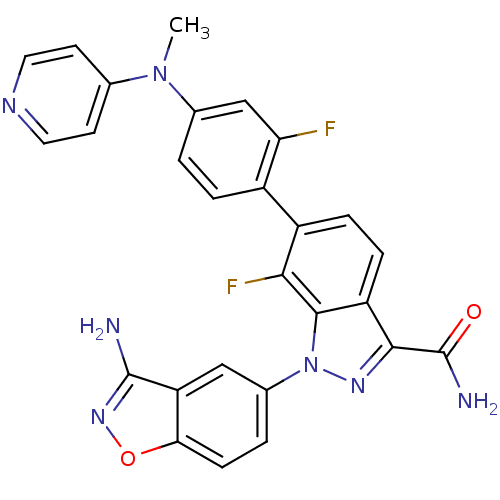
(1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...)Show SMILES CN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H19F2N7O2/c1-35(14-8-10-32-11-9-14)15-2-4-17(21(28)13-15)18-5-6-19-24(27(31)37)33-36(25(19)23(18)29)16-3-7-22-20(12-16)26(30)34-38-22/h2-13H,1H3,(H2,30,34)(H2,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231284
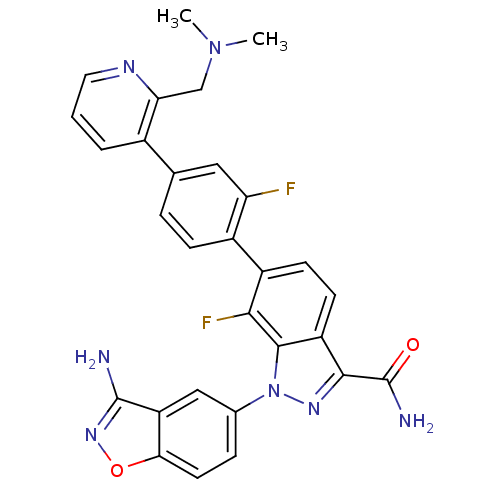
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(2-dimethyla...)Show SMILES CN(C)Cc1ncccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-23-17(4-3-11-34-23)15-5-7-18(22(30)12-15)19-8-9-20-26(29(33)39)35-38(27(20)25(19)31)16-6-10-24-21(13-16)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231271
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H26F2N8O2/c1-38(2)13-14-39(17-9-11-35-12-10-17)18-3-5-20(24(31)16-18)21-6-7-22-27(30(34)41)36-40(28(22)26(21)32)19-4-8-25-23(15-19)29(33)37-42-25/h3-12,15-16H,13-14H2,1-2H3,(H2,33,37)(H2,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231279
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(3-dimethyl...)Show SMILES CN(C)CCCN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C31H28F2N8O2/c1-39(2)14-3-15-40(18-10-12-36-13-11-18)19-4-6-21(25(32)17-19)22-7-8-23-28(31(35)42)37-41(29(23)27(22)33)20-5-9-26-24(16-20)30(34)38-43-26/h4-13,16-17H,3,14-15H2,1-2H3,(H2,34,38)(H2,35,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231283
(1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...)Show SMILES NC(=O)c1nn(-c2ccc3onc(N)c3c2)c2c(F)c(ccc12)-c1ccc(cc1F)N1CCCCC1=O Show InChI InChI=1S/C26H20F2N6O3/c27-19-12-13(33-10-2-1-3-21(33)35)4-6-15(19)16-7-8-17-23(26(30)36)31-34(24(17)22(16)28)14-5-9-20-18(11-14)25(29)32-37-20/h4-9,11-12H,1-3,10H2,(H2,29,32)(H2,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231270

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-(2'-dimethylami...)Show SMILES CN(C)Cc1ccccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H24F2N6O2/c1-37(2)15-17-5-3-4-6-19(17)16-7-9-20(24(31)13-16)21-10-11-22-27(30(34)39)35-38(28(22)26(21)32)18-8-12-25-23(14-18)29(33)36-40-25/h3-14H,15H2,1-2H3,(H2,33,36)(H2,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231272
(1-(3-amino-1,2-benzisoxazol-5-yl)-6-(4-{2-[(dimeth...)Show SMILES CN(C)Cc1nccn1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H22F2N8O2/c1-35(2)13-22-32-9-10-36(22)14-3-5-16(20(28)12-14)17-6-7-18-24(27(31)38)33-37(25(18)23(17)29)15-4-8-21-19(11-15)26(30)34-39-21/h3-12H,13H2,1-2H3,(H2,30,34)(H2,31,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231269
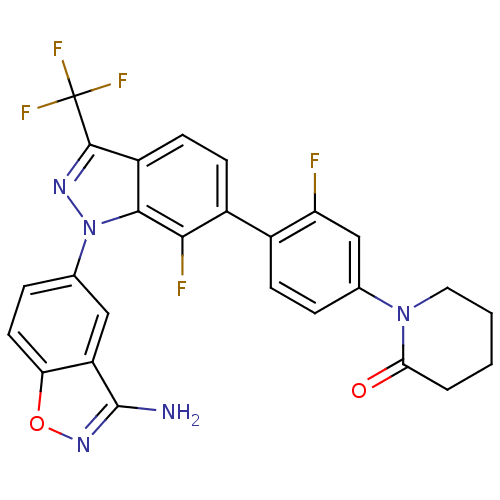
(1-{4-[1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-3-...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2ccc(c(F)c12)-c1ccc(cc1F)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C26H18F5N5O2/c27-19-12-13(35-10-2-1-3-21(35)37)4-6-15(19)16-7-8-17-23(22(16)28)36(33-24(17)26(29,30)31)14-5-9-20-18(11-14)25(32)34-38-20/h4-9,11-12H,1-3,10H2,(H2,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231274
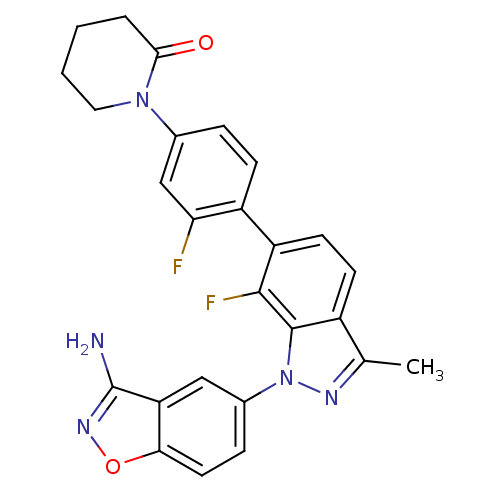
(1-{4-[1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-3-...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c(F)c(ccc12)-c1ccc(cc1F)N1CCCCC1=O Show InChI InChI=1S/C26H21F2N5O2/c1-14-17-8-9-19(18-7-5-15(13-21(18)27)32-11-3-2-4-23(32)34)24(28)25(17)33(30-14)16-6-10-22-20(12-16)26(29)31-35-22/h5-10,12-13H,2-4,11H2,1H3,(H2,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231286
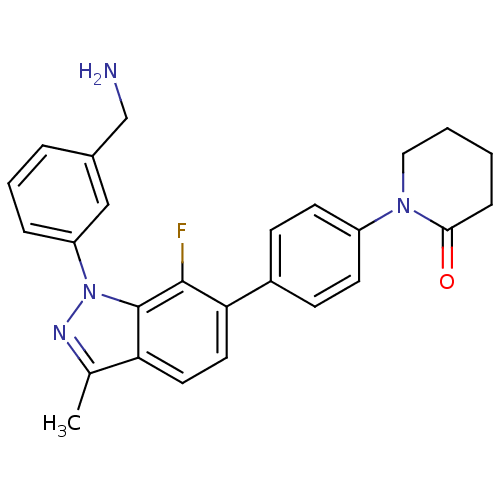
(1-{4-[1-(3-aminomethylphenyl)-7-fluoro-3-methyl-1H...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2c(F)c(ccc12)-c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H25FN4O/c1-17-22-12-13-23(19-8-10-20(11-9-19)30-14-3-2-7-24(30)32)25(27)26(22)31(29-17)21-6-4-5-18(15-21)16-28/h4-6,8-13,15H,2-3,7,14,16,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231268
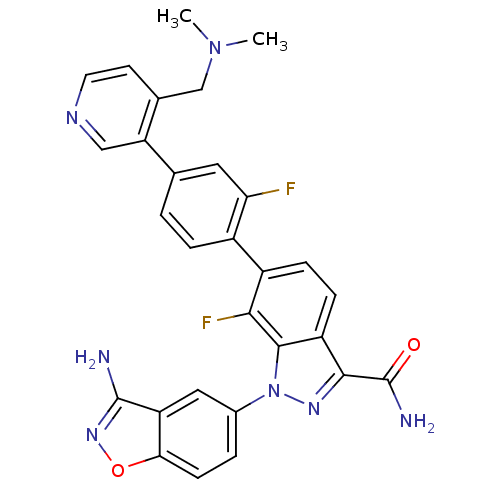
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(4-dimethyla...)Show SMILES CN(C)Cc1ccncc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-9-10-34-13-22(16)15-3-5-18(23(30)11-15)19-6-7-20-26(29(33)39)35-38(27(20)25(19)31)17-4-8-24-21(12-17)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231278
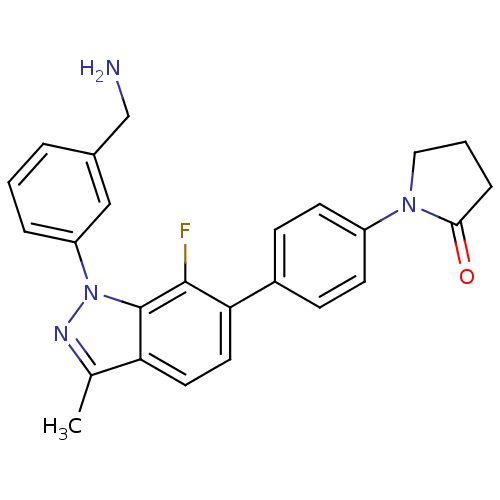
(1-{4-[1-(3-aminomethylphenyl)-7-fluoro-3-methyl-1H...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2c(F)c(ccc12)-c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C25H23FN4O/c1-16-21-11-12-22(18-7-9-19(10-8-18)29-13-3-6-23(29)31)24(26)25(21)30(28-16)20-5-2-4-17(14-20)15-27/h2,4-5,7-12,14H,3,6,13,15,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231281

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1cccnc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H26F2N8O2/c1-38(2)12-13-39(19-4-3-11-35-16-19)17-5-7-20(24(31)15-17)21-8-9-22-27(30(34)41)36-40(28(22)26(21)32)18-6-10-25-23(14-18)29(33)37-42-25/h3-11,14-16H,12-13H2,1-2H3,(H2,33,37)(H2,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231267
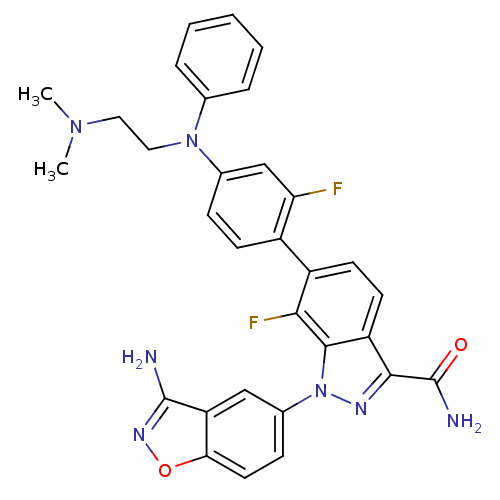
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1ccccc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C31H27F2N7O2/c1-38(2)14-15-39(18-6-4-3-5-7-18)19-8-10-21(25(32)17-19)22-11-12-23-28(31(35)41)36-40(29(23)27(22)33)20-9-13-26-24(16-20)30(34)37-42-26/h3-13,16-17H,14-15H2,1-2H3,(H2,34,37)(H2,35,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231266

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...)Show SMILES CN(C)Cc1cccnc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-4-3-11-34-25(16)15-5-7-18(22(30)12-15)19-8-9-20-26(29(33)39)35-38(27(20)24(19)31)17-6-10-23-21(13-17)28(32)36-40-23/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231273
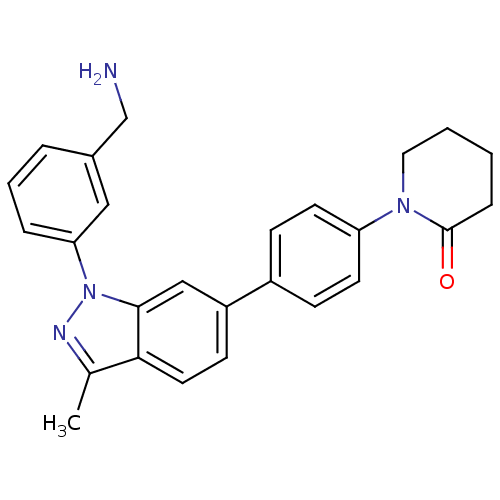
(1-{4-[1-(3-aminomethylphenyl)-3-methyl-1H-indazol-...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2cc(ccc12)-c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H26N4O/c1-18-24-13-10-21(16-25(24)30(28-18)23-6-4-5-19(15-23)17-27)20-8-11-22(12-9-20)29-14-3-2-7-26(29)31/h4-6,8-13,15-16H,2-3,7,14,17,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231265
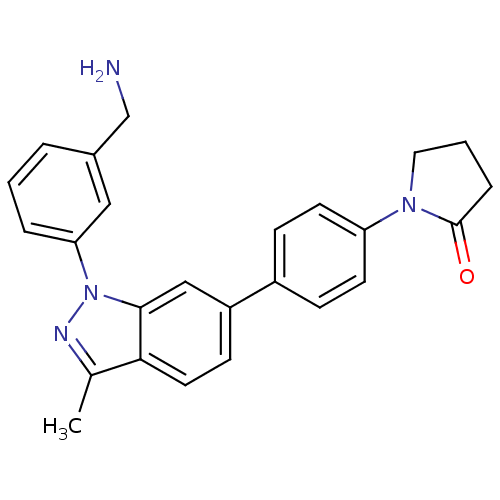
(1-{4-[1-(3-aminomethylphenyl)-3-methyl-1H-indazol-...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2cc(ccc12)-c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C25H24N4O/c1-17-23-12-9-20(19-7-10-21(11-8-19)28-13-3-6-25(28)30)15-24(23)29(27-17)22-5-2-4-18(14-22)16-26/h2,4-5,7-12,14-15H,3,6,13,16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50231277

(CHEMBL253991 | N-(4-carbamimidoylbenzyl)-2-(6-(3,5...)Show SMILES CC(C)Nc1ncc(-c2cc(N)cc(N)c2)n(CC(=O)NCc2ccc(cc2)C(N)=N)c1=O Show InChI InChI=1S/C23H28N8O2/c1-13(2)30-22-23(33)31(19(11-29-22)16-7-17(24)9-18(25)8-16)12-20(32)28-10-14-3-5-15(6-4-14)21(26)27/h3-9,11,13H,10,12,24-25H2,1-2H3,(H3,26,27)(H,28,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of factor 7a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50231276

(3-(1-(2-(4-carbamimidoylbenzylamino)-2-oxoethyl)-5...)Show SMILES CC(C)Nc1ncc(-c2cc(N)cc(c2)C(O)=O)n(CC(=O)NCc2ccc(cc2)C(N)=N)c1=O Show InChI InChI=1S/C24H27N7O4/c1-13(2)30-22-23(33)31(12-20(32)28-10-14-3-5-15(6-4-14)21(26)27)19(11-29-22)16-7-17(24(34)35)9-18(25)8-16/h3-9,11,13H,10,12,25H2,1-2H3,(H3,26,27)(H,28,32)(H,29,30)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of factor 7a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14597

(2-[3-Amino-3-fluoro-4-(isopropylamino)-1,1-bipheny...)Show SMILES CC(C)Nc1ccc(-c2cccc(N)c2)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1F Show InChI InChI=1S/C25H28FN5O/c1-15(2)31-22-11-10-20(18-4-3-5-19(27)12-18)21(24(22)26)13-23(32)30-14-16-6-8-17(9-7-16)25(28)29/h3-12,15,31H,13-14,27H2,1-2H3,(H3,28,29)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of factor 7a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data