

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50254017 (6-((4-fluorophenyl)(1H-imidazol-1-yl)methyl)benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50254016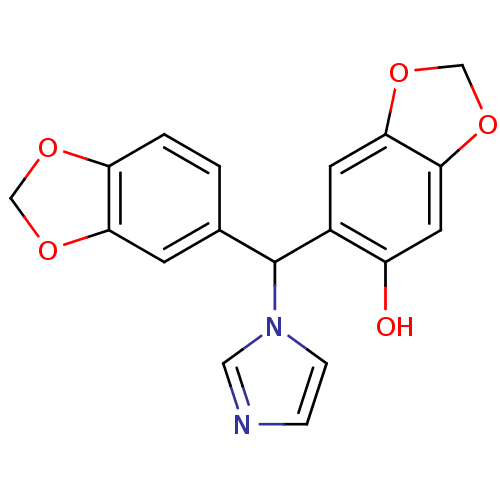 (6-(benzo[d][1,3]dioxol-5-yl(1H-imidazol-1-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50254015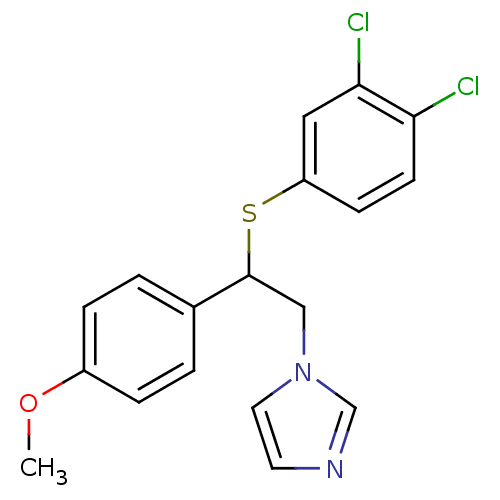 (1-(2-(3,4-dichlorophenylthio)-2-(4-methoxyphenyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265438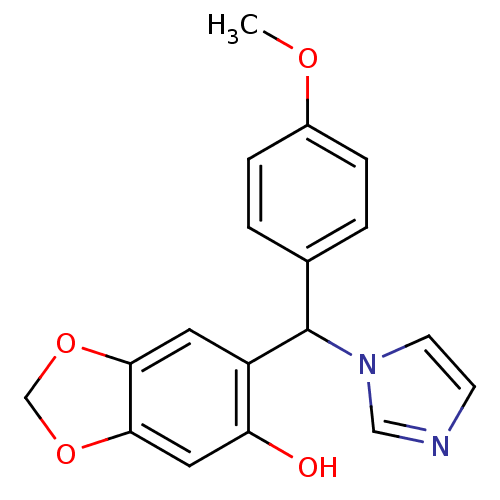 (6-((1H-imidazol-1-yl)(4-methoxyphenyl)methyl)benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265464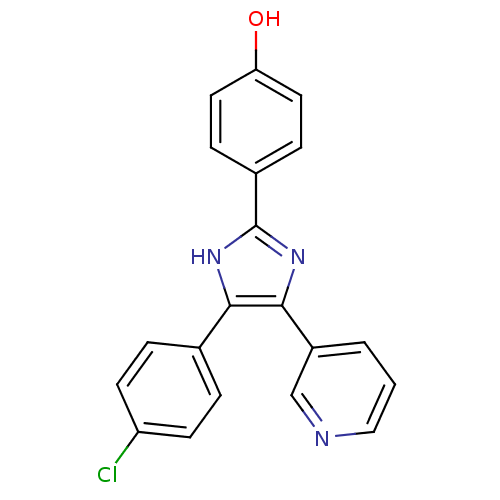 (4-(5-(4-chlorophenyl)-4-(pyridin-3-yl)-1H-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265443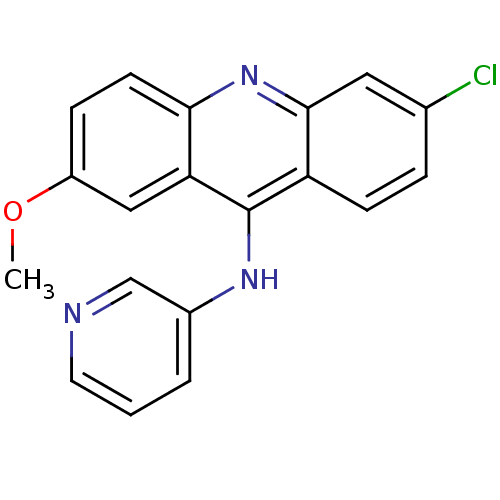 (6-chloro-2-methoxy-N-(pyridin-3-yl)acridin-9-amine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265440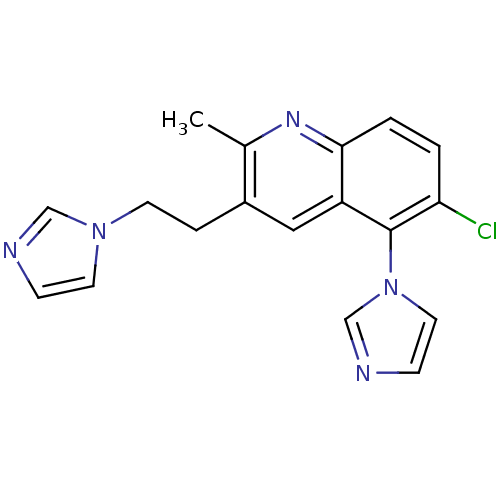 (3-(2-(1H-imidazol-1-yl)ethyl)-6-chloro-5-(1H-imida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 33.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265439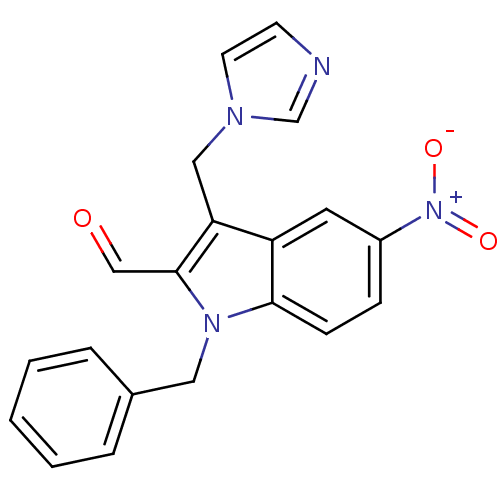 (3-((1H-imidazol-1-yl)methyl)-1-benzyl-5-nitro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 52.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50241148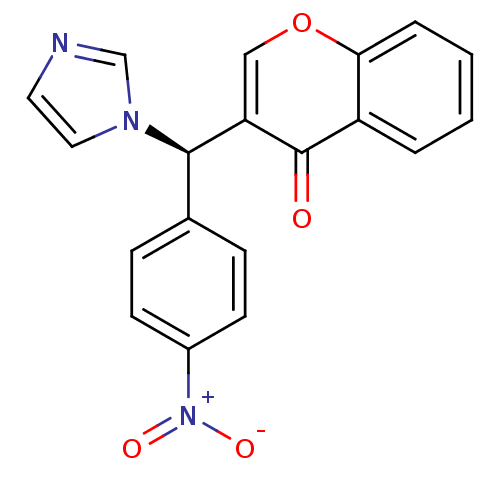 ((R)-3-((1H-imidazol-1-yl)(4-nitrophenyl)methyl)-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50254017 (6-((4-fluorophenyl)(1H-imidazol-1-yl)methyl)benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50254016 (6-(benzo[d][1,3]dioxol-5-yl(1H-imidazol-1-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50254015 (1-(2-(3,4-dichlorophenylthio)-2-(4-methoxyphenyl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097366 (4-((1H-imidazol-1-yl)methyl)-1-nitro-4aH-xanthen-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097373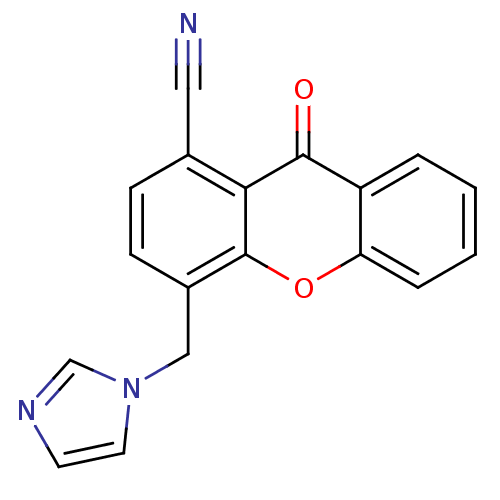 (4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191598 (3-((1H-imidazol-1-yl)methyl)-2-(4-nitrophenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265464 (4-(5-(4-chlorophenyl)-4-(pyridin-3-yl)-1H-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9916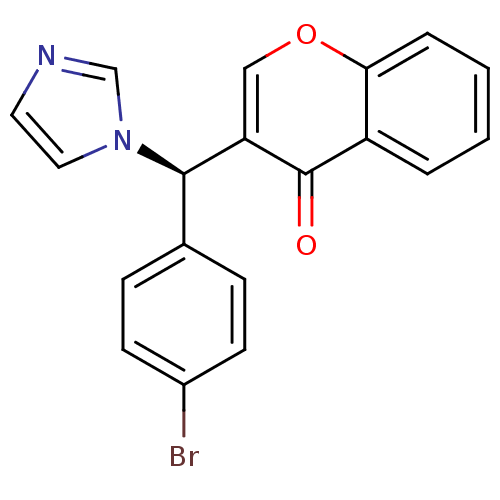 (3-[(R)-(4-bromophenyl)(1H-imidazol-1-yl)methyl]-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9475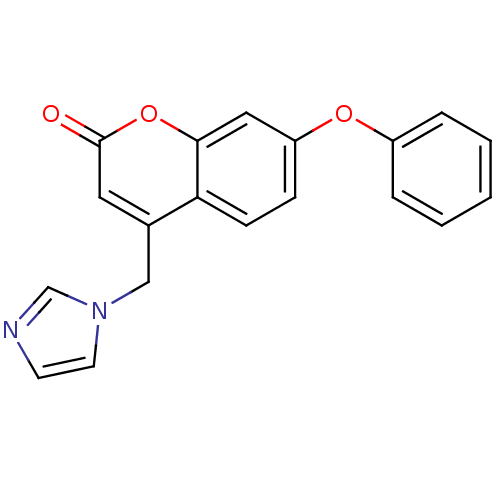 (4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265438 (6-((1H-imidazol-1-yl)(4-methoxyphenyl)methyl)benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191602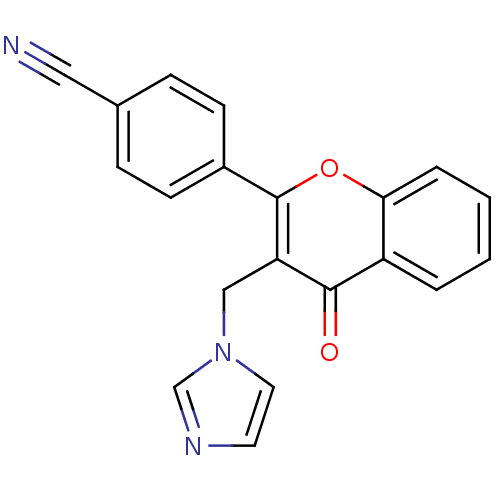 (4'-cyano-3-(imidazolylmethyl)flavone | 4-(3-((1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191599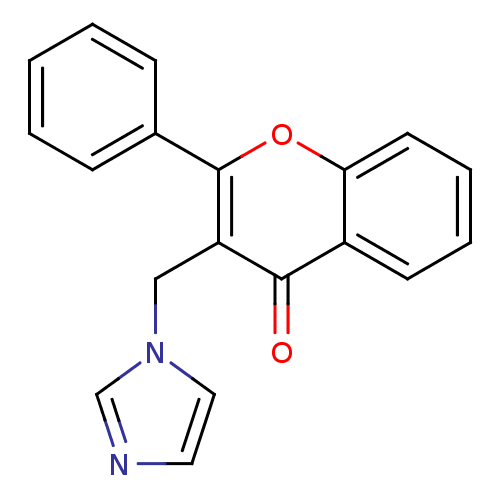 (3-((1H-imidazol-1-yl)methyl)-2-phenyl-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9465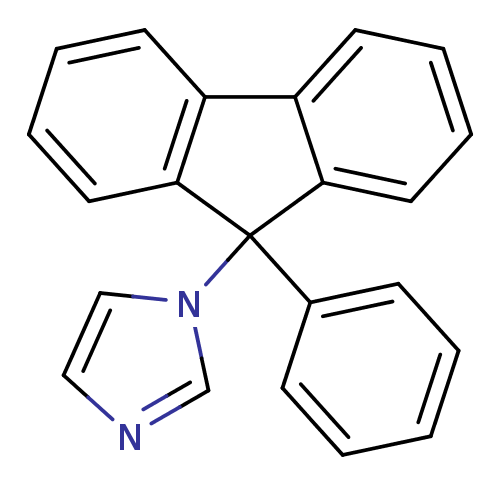 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265443 (6-chloro-2-methoxy-N-(pyridin-3-yl)acridin-9-amine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191600 (3-((1H-imidazol-1-yl)methyl)-2-(4-methoxyphenyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097367 (1-Bromo-4-imidazol-1-ylmethyl-xanthen-9-one | 4-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265543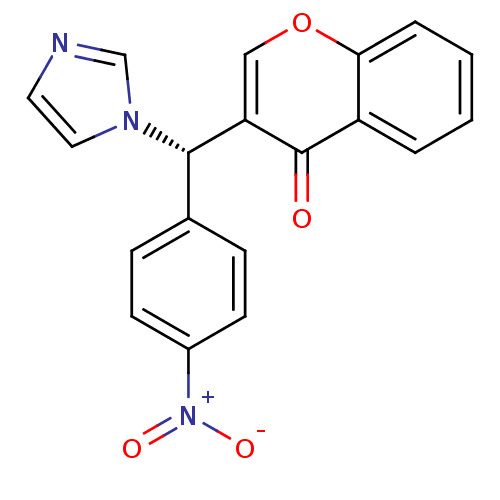 ((S)-3-((1H-imidazol-1-yl)(4-nitrophenyl)methyl)-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265440 (3-(2-(1H-imidazol-1-yl)ethyl)-6-chloro-5-(1H-imida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097369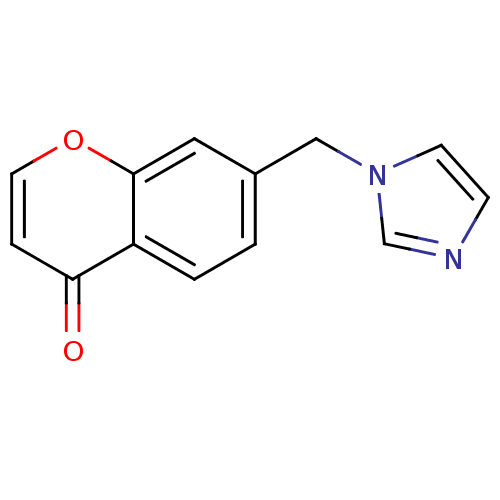 (7-((1H-imidazol-1-yl)methyl)-4H-chromen-4-one | 7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9483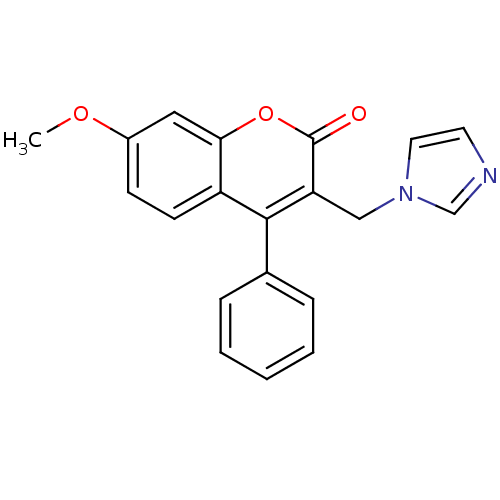 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-4-phenyl-2H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9918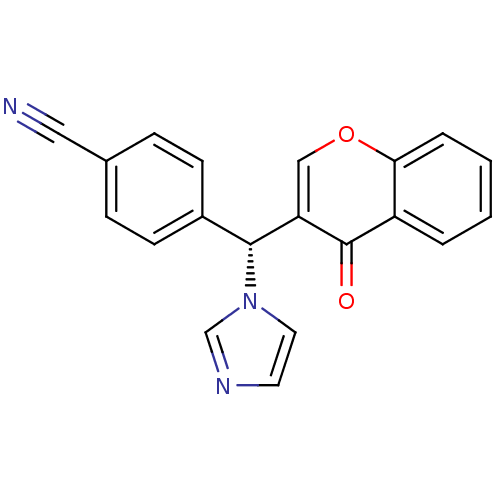 (4-[(R)-1H-imidazol-1-yl(4-oxo-4H-chromen-3-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9477 (6-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9477 (6-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9485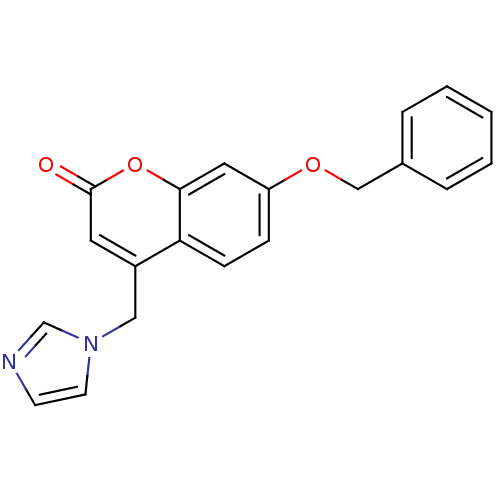 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9476 (5-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10025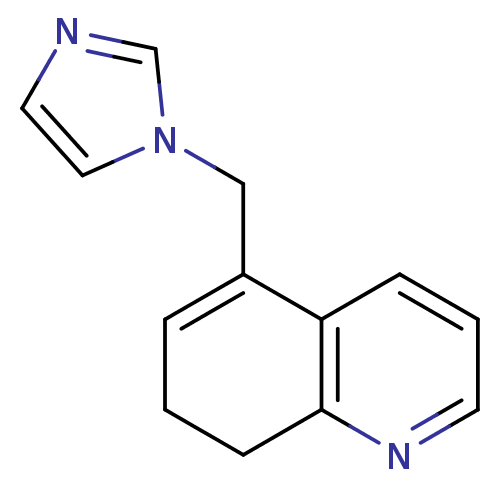 (5-(1H-imidazol-1-ylmethyl)-7,8-dihydroquinoline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265439 (3-((1H-imidazol-1-yl)methyl)-1-benzyl-5-nitro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9454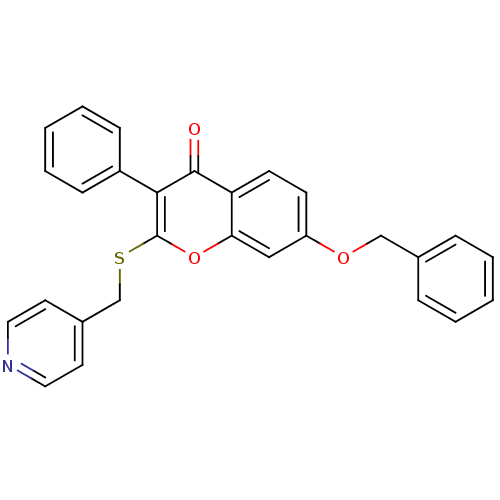 (7-(benzyloxy)-3-phenyl-2-[(pyridin-4-ylmethyl)sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50265579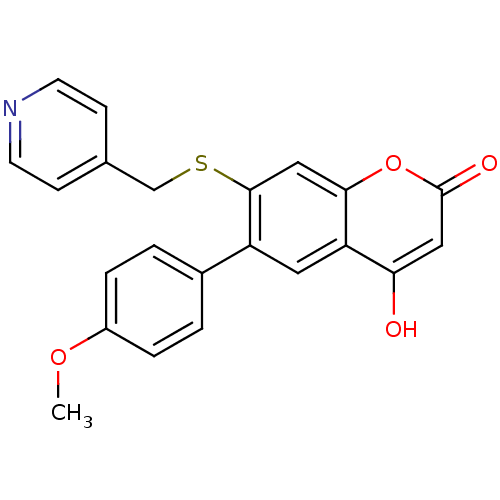 (7-hydroxy-3-(4-methoxyphenyl)-2-(pyridin-4-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10031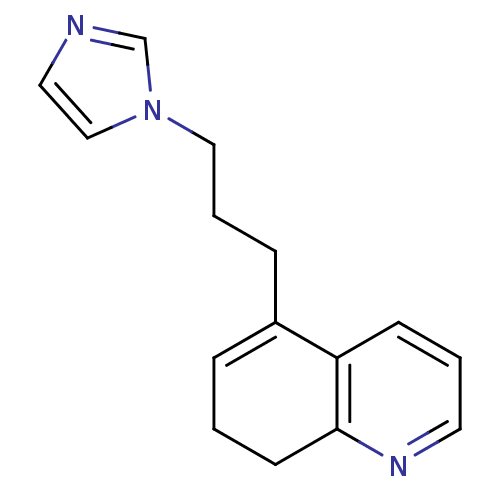 (5-[3-(1H-imidazol-1-yl)propyl]-7,8-dihydroquinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097368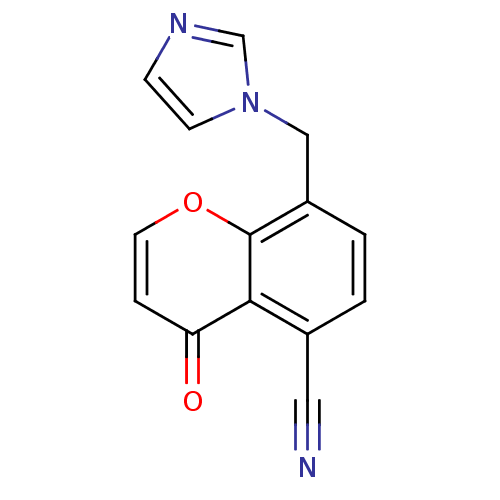 (8-((1H-imidazol-1-yl)methyl)-4-oxo-4H-chromene-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9471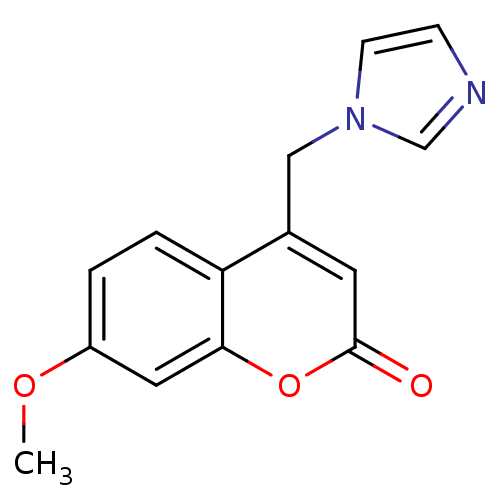 (4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9458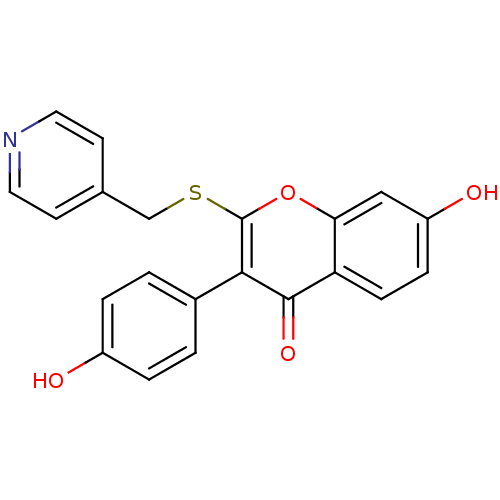 (7-Hydroxy-3-(4-hydroxyphenyl)-2-[(4-pyridylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9917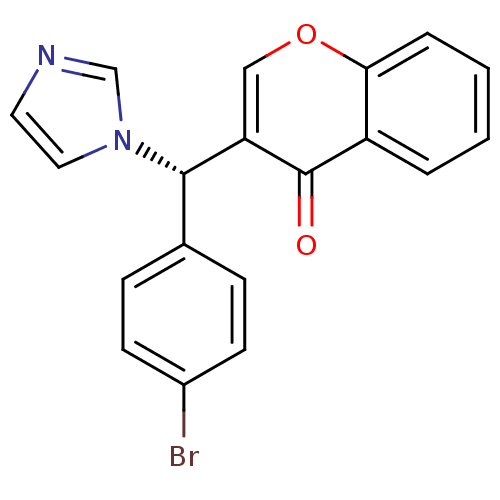 (3-[(S)-(4-bromophenyl)(1H-imidazol-1-yl)methyl]-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10028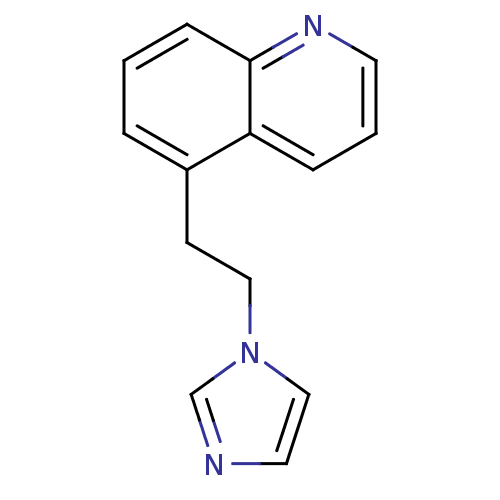 (5-[2-(1H-imidazol-1-yl)ethyl]quinoline | 5-[2-(Imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097364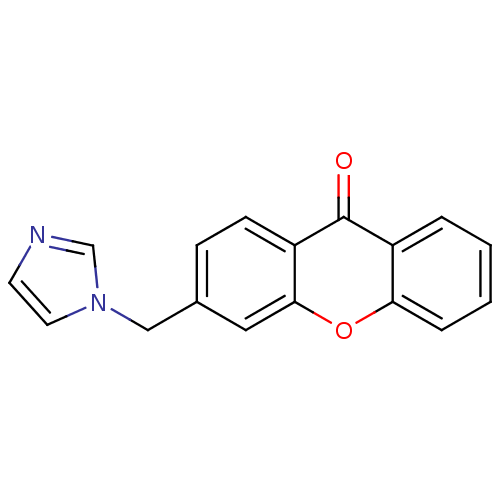 (3-((1H-imidazol-1-yl)methyl)-9H-xanthen-9-one | 3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191597 (3-((1H-imidazol-1-yl)methyl)-2-(4-bromophenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191603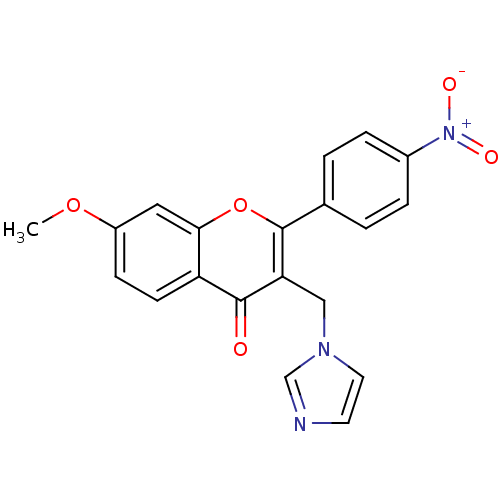 (3-((1H-imidazol-1-yl)methyl)-7-methoxy-2-(4-nitrop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10027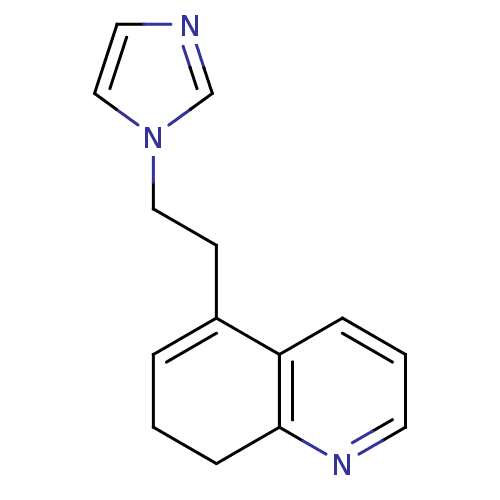 (5-[2-(1H-imidazol-1-yl)ethyl]-7,8-dihydroquinoline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |