

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50346552 (CHEMBL1797936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of synthetic VMA-tagged p300 (1287 to 1652 residues) (unknown origin) expressed in Escherichia coli BL21(RIL)-DE3 cells using H4-15 peptid... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529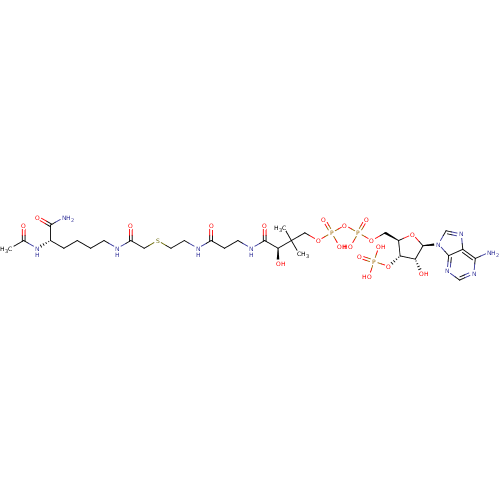 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal GST-tagged p300 (1195 to 1673 residues) expressed in competent Escherichia coli DH5alpha cells using histo... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151655 (CHEMBL3774787) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal GST-tagged p300 (1195 to 1673 residues) expressed in competent Escherichia coli DH5alpha cells using histo... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151656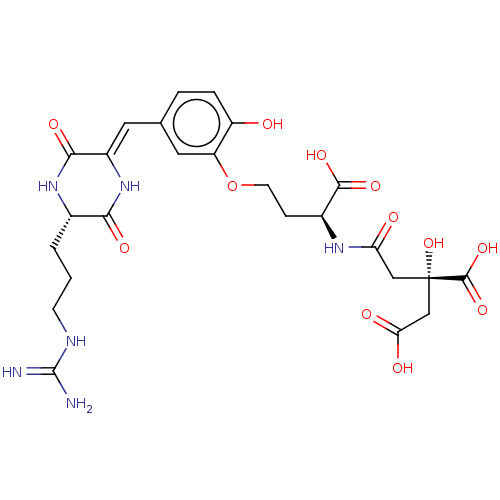 (CHEMBL3774491) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal GST-tagged p300 (1195 to 1673 residues) expressed in competent Escherichia coli DH5alpha cells using histo... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant full length p300 (unknown origin) using N-terminal H4-20 peptide substrate incubated for 7 mins by radiometric gel assay in... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151651 (CHEMBL3775655) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human recombinant p300 catalytic domain (1284 to 1673 residues) expressed in Escherichia coli using H4-8 peptide substrate by radiometr... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50151650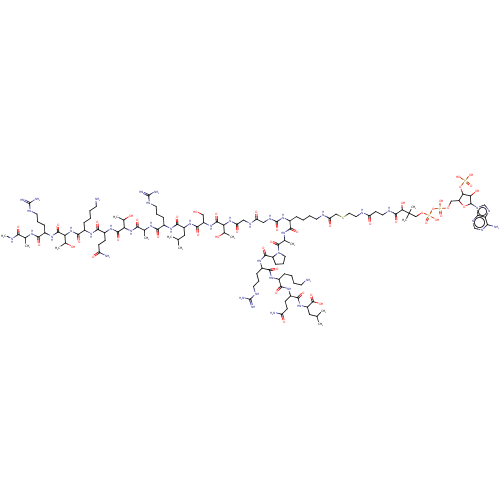 (CHEMBL3775229) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PCAF expressed in baculovirus expression system using histone substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged p300 expressed in baculovirus expression system using histone substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50346552 (CHEMBL1797936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of synthetic VMA-tagged p300 (1287 to 1652 residues) (unknown origin) expressed in Escherichia coli BL21(RIL)-DE3 cells using H4-15 peptid... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151658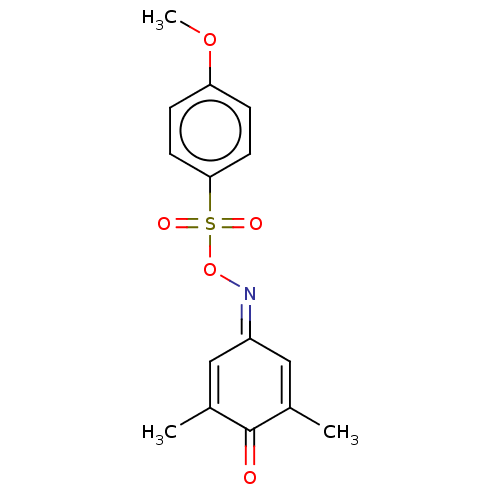 (CHEMBL3775671) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant p300 catalytic domain (unknown origin) using N-terminal histone H3 substrate by orthogonal fluorescence assay in presence o... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50012070 (5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged p300 catalytic domain (1284 to1673 residues) (unknown origin) expressed in baculovirus expression system using ... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT5 (Homo sapiens (Human)) | BDBM50151660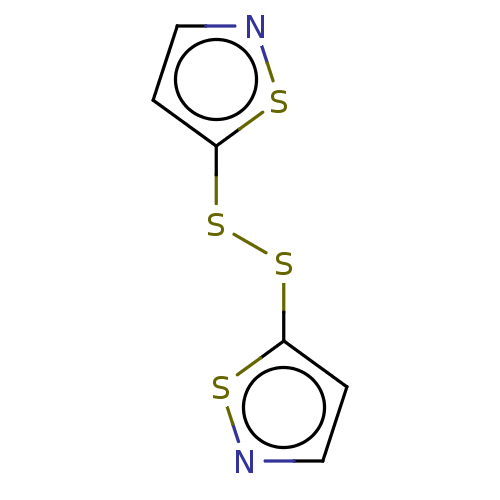 (CHEMBL3774630) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged TIP60 (unknown origin) expressed in baculovirus-infected insect cell system using histone substrate i... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50240436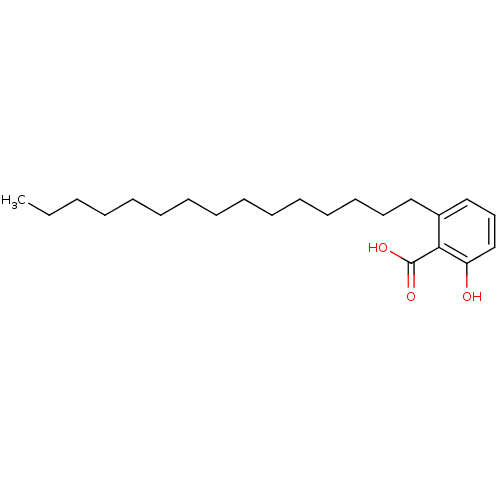 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-hydroxy-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant p300 catalytic domain (unknown origin) using N-terminal histone H3 substrate by radiometric filter binding assay in presenc... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50443249 (Ischemin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of p53-CBP bromodomain interaction in human U2OS cells assessed as inhibition of p21 activation incubated overnight followed by doxorubici... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50240436 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-hydroxy-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PCAF expressed in baculovirus infected Sf21 cells using N-terminal H3 peptide substrate after... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151654 (CHEMBL3775787) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant His6-tagged p300 (unknown origin) expressed in baculovirus infected sf21 cells using histone substrate after 10... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50151653 (CHEMBL3774884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PCAF expressed in baculovirus infected Sf21 cells using N-terminal H3 peptide substrate after... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human p300 catalytic domain (1284 to 1673 residues) using histone H3 peptide substrate after 1 hr by liquid scintillation c... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151653 (CHEMBL3774884) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged p300 expressed in baculovirus infected Sf21 cells using N-terminal H3 peptide substrate after... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50078850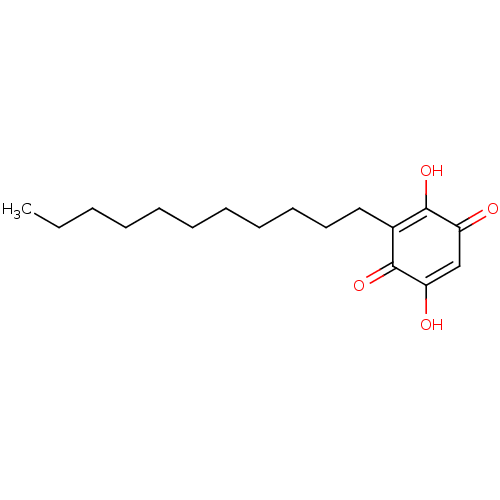 (CHEBI:4778 | Embelin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant FLAG-tagged PCAF (unknown origin) expressed in baculovirus expression system using histone substrate after 10 m... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50240436 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-hydroxy-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged p300 expressed in baculovirus infected Sf21 cells using N-terminal H3 peptide substrate after... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50346552 (CHEMBL1797936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged p300 (1195 to 1673 residues) (unknown origin) expressed in competent Escherichia coli BL21-CodonPlus(DE3)-RIL cells using h... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50012070 (5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant His6-tagged p300 (unknown origin) expressed in baculovirus expression system using histone substrate after 10 m... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant p300 (unknown origin) expressed in baculovirus expression system using histone substrate after 10 mins by liquid scintillat... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant CBP (unknown origin) expressed in baculovirus expression system using histone substrate after 10 mins by liquid scintillati... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged p300 (unknown origin) using histone H4 peptide substrate assessed as reduction in NADH formation by spectrophoto... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2A (Homo sapiens (Human)) | BDBM50151658 (CHEMBL3775671) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of GCN5 (unknown origin) using N-terminal histone H3 substrate by radiometric filter binding based phenotypic screen in presence of [3H]ac... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50240436 (2-Hydroxy-6-pentadecyl-benzoic acid | 2-hydroxy-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human PCAF catalytic domain (492 to 658 residues) using histone H3 peptide substrate after 1 hr by liquid scintillation cou... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50151658 (CHEMBL3775671) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of PCAF (unknown origin) using N-terminal histone H3 substrate by radiometric filter binding based phenotypic screen in presence of [3H]ac... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50151660 (CHEMBL3774630) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged PCAF (unknown origin) expressed in Escherichia coli cells using histone substrate incubated for 10 mins by radio... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged CBP (unknown origin) using histone H4 peptide substrate assessed as reduction in NADH formation by spectrophotom... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50012070 (5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant PCAF (unknown origin) expressed in baculovirus expression system using histone substrate after 10 mins by liquid scintillat... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151660 (CHEMBL3774630) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged p300 (unknown origin) expressed in Escherichia coli cells using histone substrate incubated for 10 mins by radio... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant PCAF (unknown origin) using histone H4 peptide substrate assessed as reduction in NADH formation by spectrophotometric anal... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT5 (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant TIP60 (unknown origin) using histone H4 peptide substrate assessed as reduction in NADH formation by spectrophotometric ana... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2A (Homo sapiens (Human)) | BDBM50151657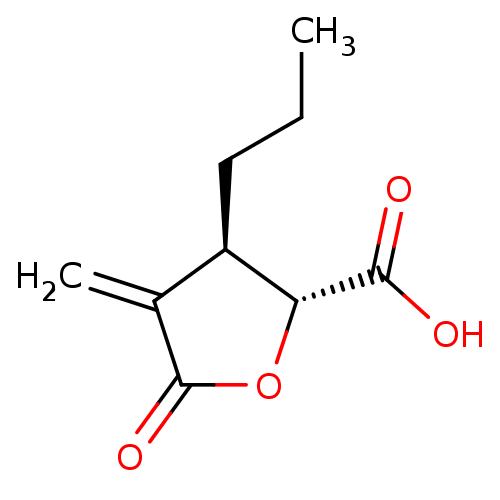 (CHEMBL3775750) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GCN5 expressed in bacterial system using histone H3 as substrate preincubated for 20 mins followed by subs... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50151659 (CHEMBL3775244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of PCAF (unknown origin) using N-terminal histone H3-20 peptide substrate incubated for 3.5 mins by liquid scintillation counting method i... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151658 (CHEMBL3775671) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant p300 catalytic domain (unknown origin) using N-terminal histone H3 substrate by radiometric filter binding based phenotypic... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT5 (Homo sapiens (Human)) | BDBM50151659 (CHEMBL3775244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length TIP60 (unknown origin) expressed in Escherichia coli using N-terminal histone H4-20 peptide substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151659 (CHEMBL3775244) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of p300 (unknown origin) using N-terminal histone H4-20 peptide substrate incubated for 10 mins by liquid scintillation counting method in... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Spermidine synthase (Sus scrofa) | BDBM50151659 (CHEMBL3775244) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of yeast ESA1 using N-terminal histone H3 substrate by radiometric filter binding based virtual screen in presence of [3H]acetyl-CoA | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PCAF expressed in baculovirus expression system using histone substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151650 (CHEMBL3775229) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged p300 expressed in baculovirus expression system using histone substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151652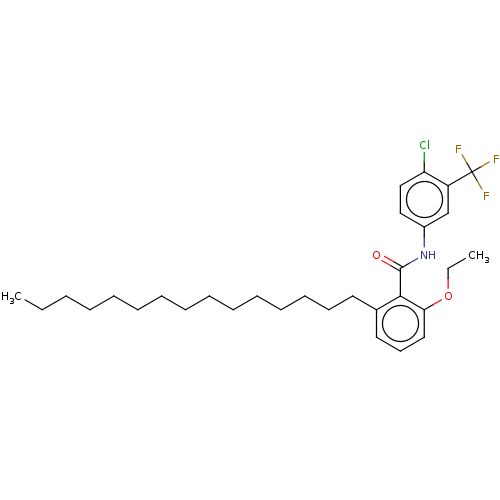 (CHEMBL3774466) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human His6-tagged p300 expressed in baculovirus infected Sf21 cells using N-terminal H3 peptide substrate after... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50151657 (CHEMBL3775750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant CBP (unknown origin) expressed in baculovirus expression system using histone H3 as substrate preincubated for 20 mins foll... | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50151663 (CHEMBL3774655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human CREBBP expressed in competent escherichia coli BL21(DE3)-R3-pRARE2 cells by isothermal titration calorimetry | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50151662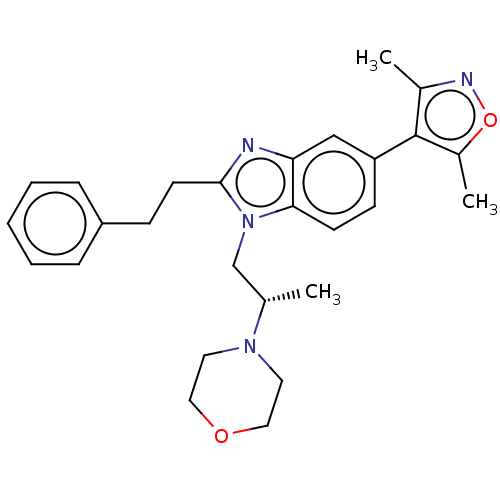 (CHEMBL3775371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human CREBBP expressed in competent escherichia coli BL21(DE3)-R3-pRARE2 cells by isothermal titration calorimetry | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50151661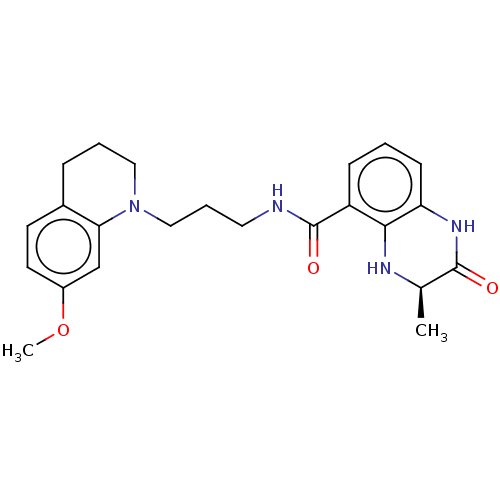 (CHEMBL3752296) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human BRD4 by isothermal titration calorimetry | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50151661 (CHEMBL3752296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human His6-tagged CREBBP expressed in competent Escherichia coli BL21 (DE3) cells by isothermal titration calorimetry | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50151663 (CHEMBL3774655) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human p300 expressed in competent escherichia coli BL21(DE3)-R3-pRARE2 cells by isothermal titration calorimetry | J Med Chem 59: 1249-70 (2016) Article DOI: 10.1021/acs.jmedchem.5b01502 BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||