 Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50031501
Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50031501 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314332
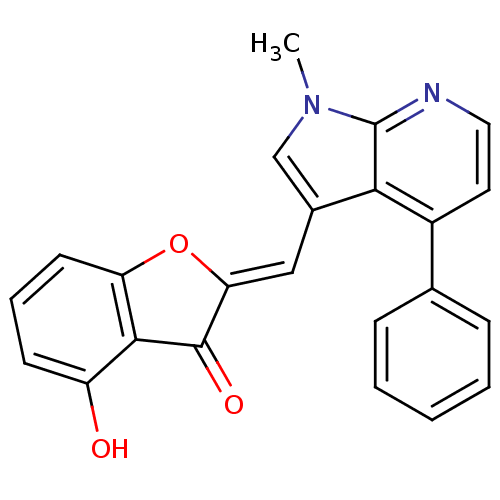
(4-hydroxy-2-((1-methyl-4-phenyl-1H-pyrrolo[2,3-b]p...)Show SMILES Cn1cc(\C=C2/Oc3cccc(O)c3C2=O)c2c(ccnc12)-c1ccccc1 Show InChI InChI=1S/C23H16N2O3/c1-25-13-15(12-19-22(27)21-17(26)8-5-9-18(21)28-19)20-16(10-11-24-23(20)25)14-6-3-2-4-7-14/h2-13,26H,1H3/b19-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314317
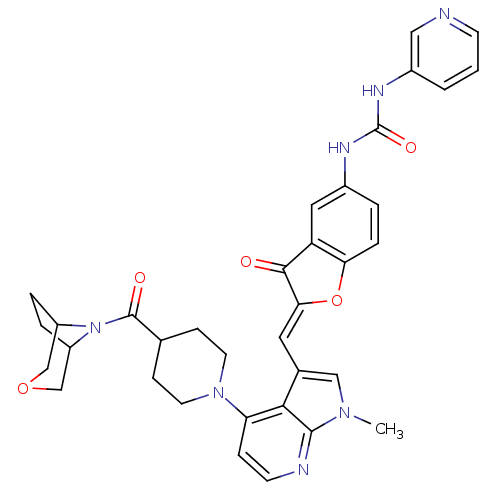
((Z)-1-(2-((4-(4-(3-oxa-8-azabicyclo[3.2.1]octane-8...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1C2CCC1COC2 Show InChI InChI=1S/C35H35N7O5/c1-40-18-22(15-30-32(43)27-16-23(4-7-29(27)47-30)38-35(45)39-24-3-2-11-36-17-24)31-28(8-12-37-33(31)40)41-13-9-21(10-14-41)34(44)42-25-5-6-26(42)20-46-19-25/h2-4,7-8,11-12,15-18,21,25-26H,5-6,9-10,13-14,19-20H2,1H3,(H2,38,39,45)/b30-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314327

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES CN(C)CCNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(N5CC6CCC(C5)O6)c34)C(=O)c2c1 Show InChI InChI=1S/C28H32N6O4/c1-32(2)11-10-30-28(36)31-18-4-7-23-21(13-18)26(35)24(38-23)12-17-14-33(3)27-25(17)22(8-9-29-27)34-15-19-5-6-20(16-34)37-19/h4,7-9,12-14,19-20H,5-6,10-11,15-16H2,1-3H3,(H2,30,31,36)/b24-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314322
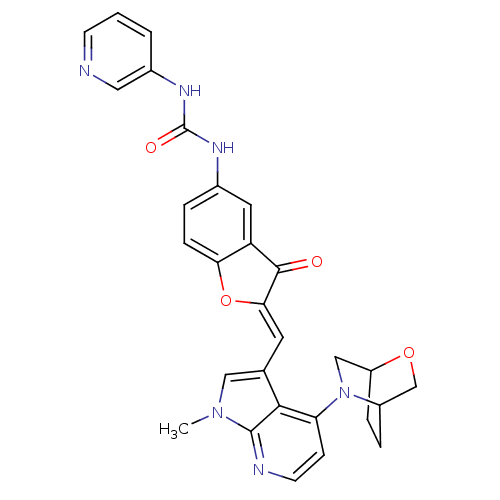
((Z)-1-(2-((4-(2-oxa-5-azabicyclo[2.2.2]octan-5-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CC2CCC1CO2 Show InChI InChI=1S/C29H26N6O4/c1-34-14-17(26-23(8-10-31-28(26)34)35-15-21-6-5-20(35)16-38-21)11-25-27(36)22-12-18(4-7-24(22)39-25)32-29(37)33-19-3-2-9-30-13-19/h2-4,7-14,20-21H,5-6,15-16H2,1H3,(H2,32,33,37)/b25-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314339

((Z)-1-(2-((4-(4-(3-methoxypyrrolidine-1-carbonyl)p...)Show SMILES COC1CCN(C1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C34H35N7O5/c1-39-19-22(16-29-31(42)26-17-23(5-6-28(26)46-29)37-34(44)38-24-4-3-11-35-18-24)30-27(7-12-36-32(30)39)40-13-8-21(9-14-40)33(43)41-15-10-25(20-41)45-2/h3-7,11-12,16-19,21,25H,8-10,13-15,20H2,1-2H3,(H2,37,38,44)/b29-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314342

((Z)-1-(2-((1-methyl-4-(4-(pyrrolidine-1-carbonyl)p...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C33H33N7O4/c1-38-20-22(29-26(8-12-35-31(29)38)39-15-9-21(10-16-39)32(42)40-13-2-3-14-40)17-28-30(41)25-18-23(6-7-27(25)44-28)36-33(43)37-24-5-4-11-34-19-24/h4-8,11-12,17-21H,2-3,9-10,13-16H2,1H3,(H2,36,37,43)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314335
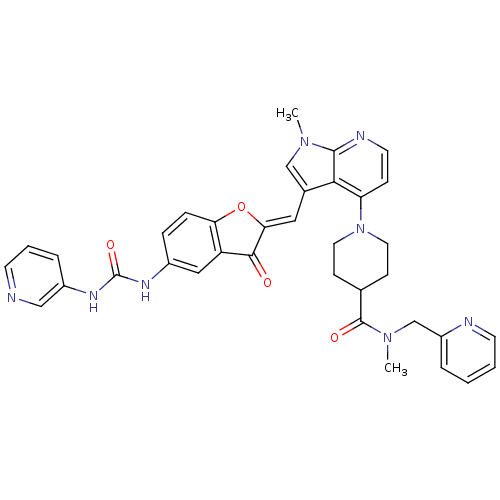
((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(Cc1ccccn1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C36H34N8O4/c1-42-21-24(18-31-33(45)28-19-25(8-9-30(28)48-31)40-36(47)41-26-7-5-13-37-20-26)32-29(10-15-39-34(32)42)44-16-11-23(12-17-44)35(46)43(2)22-27-6-3-4-14-38-27/h3-10,13-15,18-21,23H,11-12,16-17,22H2,1-2H3,(H2,40,41,47)/b31-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314338
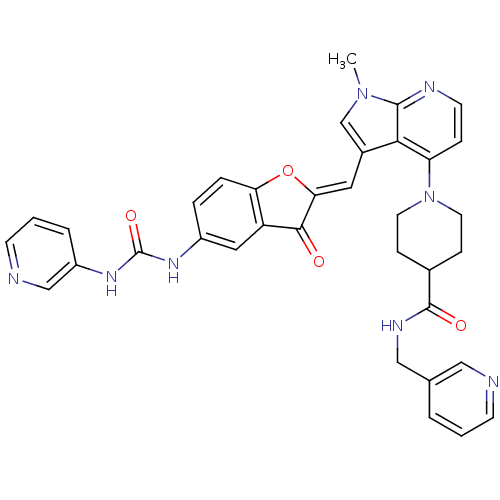
((Z)-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-ylureido)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C35H32N8O4/c1-42-21-24(16-30-32(44)27-17-25(6-7-29(27)47-30)40-35(46)41-26-5-3-12-37-20-26)31-28(8-13-38-33(31)42)43-14-9-23(10-15-43)34(45)39-19-22-4-2-11-36-18-22/h2-8,11-13,16-18,20-21,23H,9-10,14-15,19H2,1H3,(H,39,45)(H2,40,41,46)/b30-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314336

((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(Cc1ccncc1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C36H34N8O4/c1-42-22-25(18-31-33(45)28-19-26(5-6-30(28)48-31)40-36(47)41-27-4-3-12-38-20-27)32-29(9-15-39-34(32)42)44-16-10-24(11-17-44)35(46)43(2)21-23-7-13-37-14-8-23/h3-9,12-15,18-20,22,24H,10-11,16-17,21H2,1-2H3,(H2,40,41,47)/b31-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314329

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES CNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(N5CC6CCC(C5)O6)c34)C(=O)c2c1 Show InChI InChI=1S/C25H25N5O4/c1-26-25(32)28-15-3-6-20-18(10-15)23(31)21(34-20)9-14-11-29(2)24-22(14)19(7-8-27-24)30-12-16-4-5-17(13-30)33-16/h3,6-11,16-17H,4-5,12-13H2,1-2H3,(H2,26,28,32)/b21-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314320
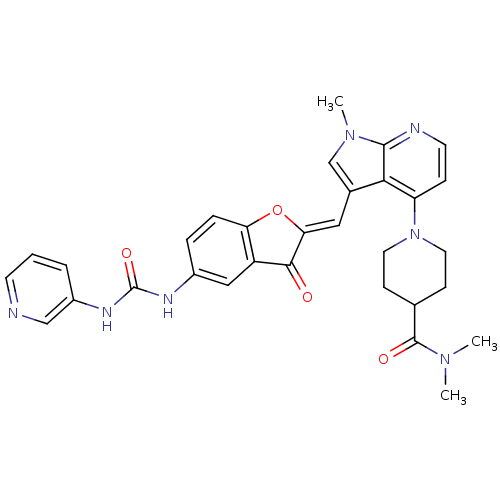
((Z)-N,N-dimethyl-1-(1-methyl-3-((3-oxo-5-(3-pyridi...)Show SMILES CN(C)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C31H31N7O4/c1-36(2)30(40)19-9-13-38(14-10-19)24-8-12-33-29-27(24)20(18-37(29)3)15-26-28(39)23-16-21(6-7-25(23)42-26)34-31(41)35-22-5-4-11-32-17-22/h4-8,11-12,15-19H,9-10,13-14H2,1-3H3,(H2,34,35,41)/b26-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314337
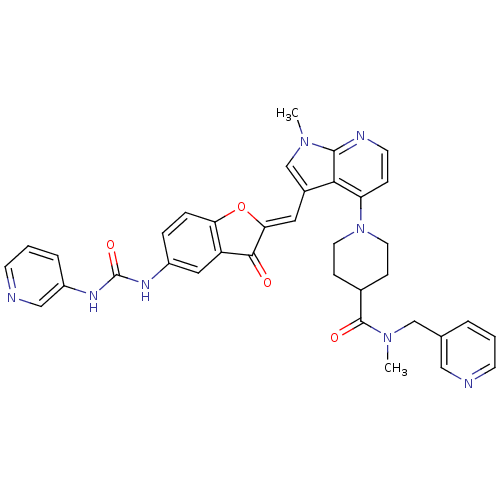
((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(Cc1cccnc1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C36H34N8O4/c1-42-22-25(17-31-33(45)28-18-26(7-8-30(28)48-31)40-36(47)41-27-6-4-13-38-20-27)32-29(9-14-39-34(32)42)44-15-10-24(11-16-44)35(46)43(2)21-23-5-3-12-37-19-23/h3-9,12-14,17-20,22,24H,10-11,15-16,21H2,1-2H3,(H2,40,41,47)/b31-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314340
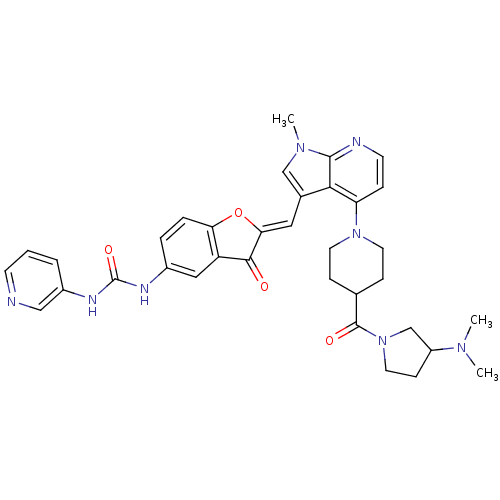
((Z)-1-(2-((4-(4-(3-(dimethylamino)pyrrolidine-1-ca...)Show SMILES CN(C)C1CCN(C1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C35H38N8O4/c1-40(2)26-11-16-43(21-26)34(45)22-9-14-42(15-10-22)28-8-13-37-33-31(28)23(20-41(33)3)17-30-32(44)27-18-24(6-7-29(27)47-30)38-35(46)39-25-5-4-12-36-19-25/h4-8,12-13,17-20,22,26H,9-11,14-16,21H2,1-3H3,(H2,38,39,46)/b30-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314333
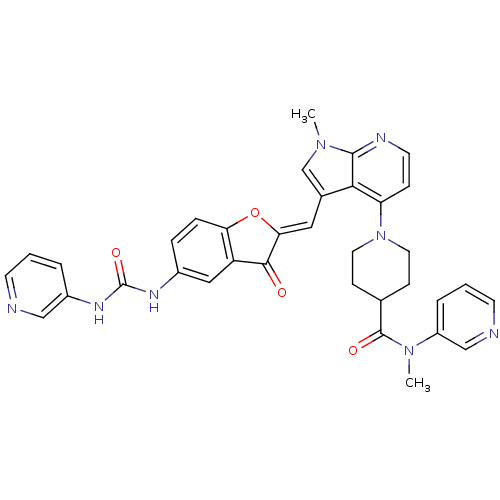
((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12)c1cccnc1 Show InChI InChI=1S/C35H32N8O4/c1-41-21-23(17-30-32(44)27-18-24(7-8-29(27)47-30)39-35(46)40-25-5-3-12-36-19-25)31-28(9-14-38-33(31)41)43-15-10-22(11-16-43)34(45)42(2)26-6-4-13-37-20-26/h3-9,12-14,17-22H,10-11,15-16H2,1-2H3,(H2,39,40,46)/b30-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314325

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CC2CCC(C1)O2 Show InChI InChI=1S/C29H26N6O4/c1-34-14-17(26-23(8-10-31-28(26)34)35-15-20-5-6-21(16-35)38-20)11-25-27(36)22-12-18(4-7-24(22)39-25)32-29(37)33-19-3-2-9-30-13-19/h2-4,7-14,20-21H,5-6,15-16H2,1H3,(H2,32,33,37)/b25-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314316
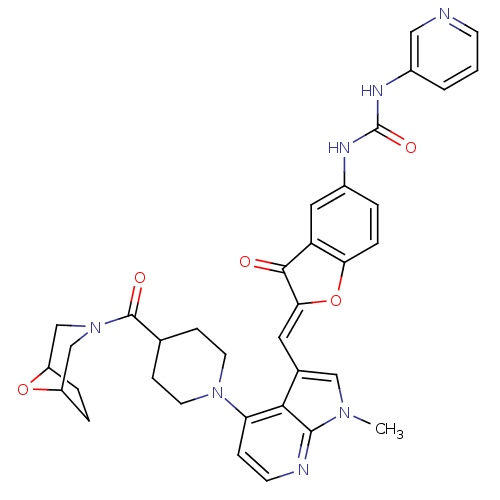
((Z)-1-(2-((4-(4-(8-oxa-3-azabicyclo[3.2.1]octane-3...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CC2CCC(C1)O2 Show InChI InChI=1S/C35H35N7O5/c1-40-18-22(15-30-32(43)27-16-23(4-7-29(27)47-30)38-35(45)39-24-3-2-11-36-17-24)31-28(8-12-37-33(31)40)41-13-9-21(10-14-41)34(44)42-19-25-5-6-26(20-42)46-25/h2-4,7-8,11-12,15-18,21,25-26H,5-6,9-10,13-14,19-20H2,1H3,(H2,38,39,45)/b30-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314318
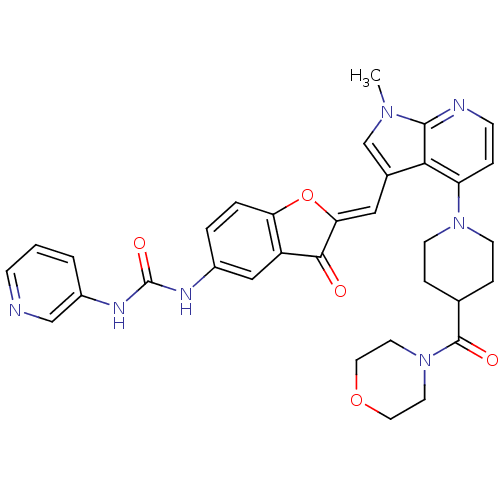
((Z)-1-(2-((1-methyl-4-(4-(morpholine-4-carbonyl)pi...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CCOCC1 Show InChI InChI=1S/C33H33N7O5/c1-38-20-22(17-28-30(41)25-18-23(4-5-27(25)45-28)36-33(43)37-24-3-2-9-34-19-24)29-26(6-10-35-31(29)38)39-11-7-21(8-12-39)32(42)40-13-15-44-16-14-40/h2-6,9-10,17-21H,7-8,11-16H2,1H3,(H2,36,37,43)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314327

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES CN(C)CCNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(N5CC6CCC(C5)O6)c34)C(=O)c2c1 Show InChI InChI=1S/C28H32N6O4/c1-32(2)11-10-30-28(36)31-18-4-7-23-21(13-18)26(35)24(38-23)12-17-14-33(3)27-25(17)22(8-9-29-27)34-15-19-5-6-20(16-34)37-19/h4,7-9,12-14,19-20H,5-6,10-11,15-16H2,1-3H3,(H2,30,31,36)/b24-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314324
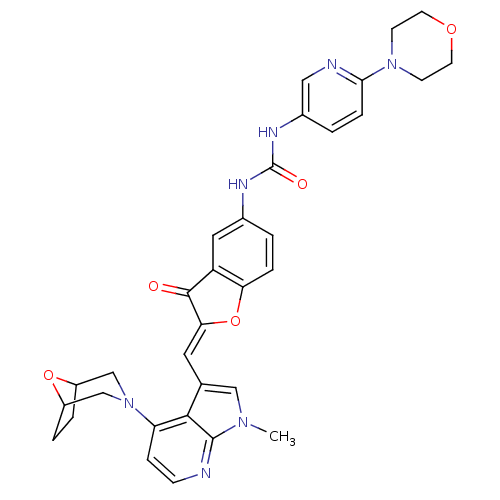
((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4ccc(nc4)N4CCOCC4)cc3C2=O)c2c(ccnc12)N1CC2CCC(C1)O2 Show InChI InChI=1S/C33H33N7O5/c1-38-17-20(30-26(8-9-34-32(30)38)40-18-23-4-5-24(19-40)44-23)14-28-31(41)25-15-21(2-6-27(25)45-28)36-33(42)37-22-3-7-29(35-16-22)39-10-12-43-13-11-39/h2-3,6-9,14-17,23-24H,4-5,10-13,18-19H2,1H3,(H2,36,37,42)/b28-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314331
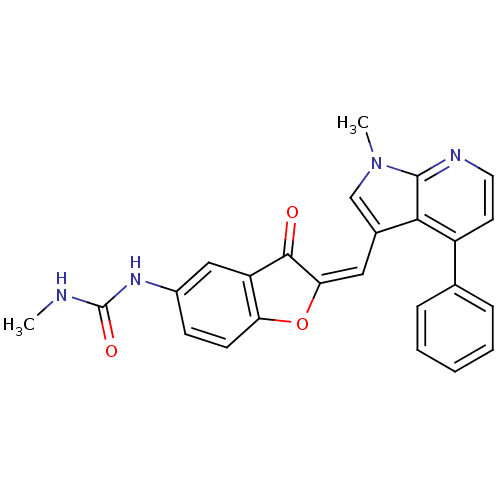
((Z)-1-methyl-3-(2-((1-methyl-4-phenyl-1H-pyrrolo[2...)Show SMILES CNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(-c5ccccc5)c34)C(=O)c2c1 Show InChI InChI=1S/C25H20N4O3/c1-26-25(31)28-17-8-9-20-19(13-17)23(30)21(32-20)12-16-14-29(2)24-22(16)18(10-11-27-24)15-6-4-3-5-7-15/h3-14H,1-2H3,(H2,26,28,31)/b21-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314321
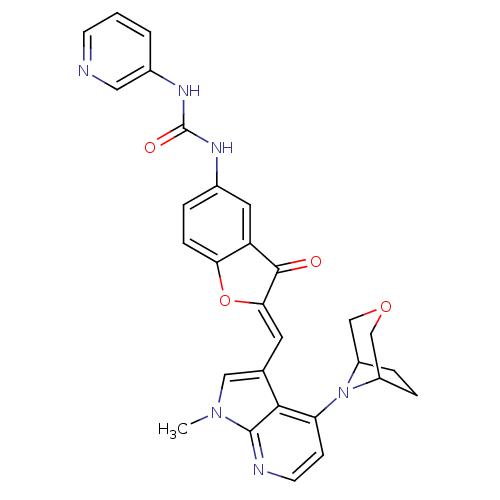
((Z)-1-(2-((4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1C2CCC1COC2 Show InChI InChI=1S/C29H26N6O4/c1-34-14-17(26-23(8-10-31-28(26)34)35-20-5-6-21(35)16-38-15-20)11-25-27(36)22-12-18(4-7-24(22)39-25)32-29(37)33-19-3-2-9-30-13-19/h2-4,7-14,20-21H,5-6,15-16H2,1H3,(H2,32,33,37)/b25-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314341
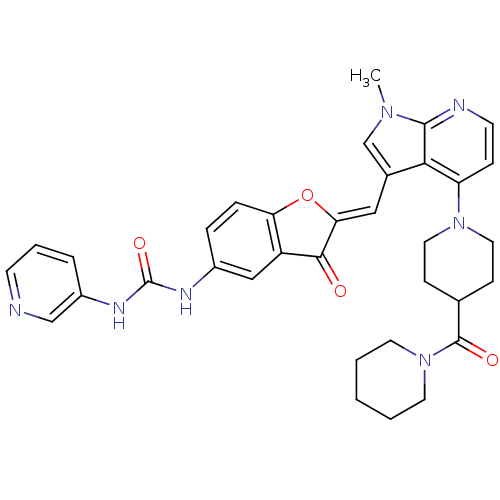
((Z)-1-(2-((1-methyl-4-(4-(piperidine-1-carbonyl)pi...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CCCCC1 Show InChI InChI=1S/C34H35N7O4/c1-39-21-23(18-29-31(42)26-19-24(7-8-28(26)45-29)37-34(44)38-25-6-5-12-35-20-25)30-27(9-13-36-32(30)39)40-16-10-22(11-17-40)33(43)41-14-3-2-4-15-41/h5-9,12-13,18-22H,2-4,10-11,14-17H2,1H3,(H2,37,38,44)/b29-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314328
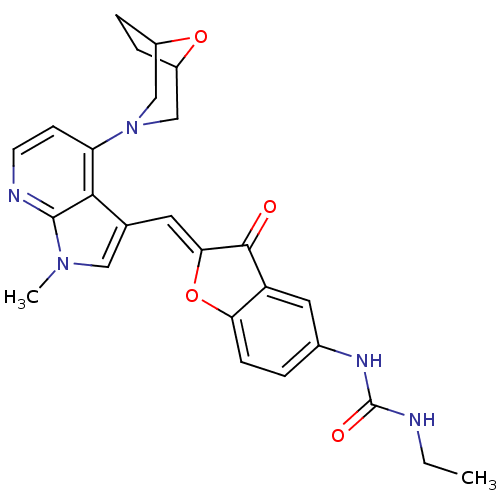
((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES CCNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(N5CC6CCC(C5)O6)c34)C(=O)c2c1 Show InChI InChI=1S/C26H27N5O4/c1-3-27-26(33)29-16-4-7-21-19(11-16)24(32)22(35-21)10-15-12-30(2)25-23(15)20(8-9-28-25)31-13-17-5-6-18(14-31)34-17/h4,7-12,17-18H,3,5-6,13-14H2,1-2H3,(H2,27,29,33)/b22-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314343
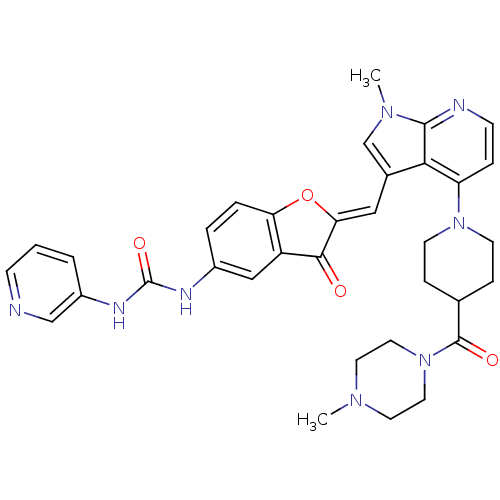
((Z)-1-(2-((1-methyl-4-(4-(4-methylpiperazine-1-car...)Show SMILES CN1CCN(CC1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C34H36N8O4/c1-39-14-16-42(17-15-39)33(44)22-8-12-41(13-9-22)27-7-11-36-32-30(27)23(21-40(32)2)18-29-31(43)26-19-24(5-6-28(26)46-29)37-34(45)38-25-4-3-10-35-20-25/h3-7,10-11,18-22H,8-9,12-17H2,1-2H3,(H2,37,38,45)/b29-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314323
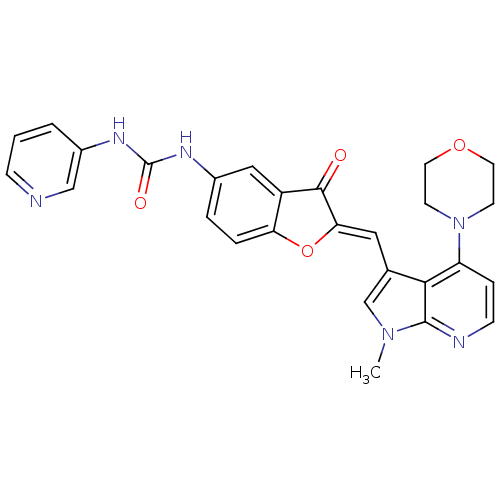
((Z)-1-(2-((1-methyl-4-morpholino-1H-pyrrolo[2,3-b]...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCOCC1 Show InChI InChI=1S/C27H24N6O4/c1-32-16-17(24-21(6-8-29-26(24)32)33-9-11-36-12-10-33)13-23-25(34)20-14-18(4-5-22(20)37-23)30-27(35)31-19-3-2-7-28-15-19/h2-8,13-16H,9-12H2,1H3,(H2,30,31,35)/b23-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314332

(4-hydroxy-2-((1-methyl-4-phenyl-1H-pyrrolo[2,3-b]p...)Show SMILES Cn1cc(\C=C2/Oc3cccc(O)c3C2=O)c2c(ccnc12)-c1ccccc1 Show InChI InChI=1S/C23H16N2O3/c1-25-13-15(12-19-22(27)21-17(26)8-5-9-18(21)28-19)20-16(10-11-24-23(20)25)14-6-3-2-4-7-14/h2-13,26H,1H3/b19-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314334
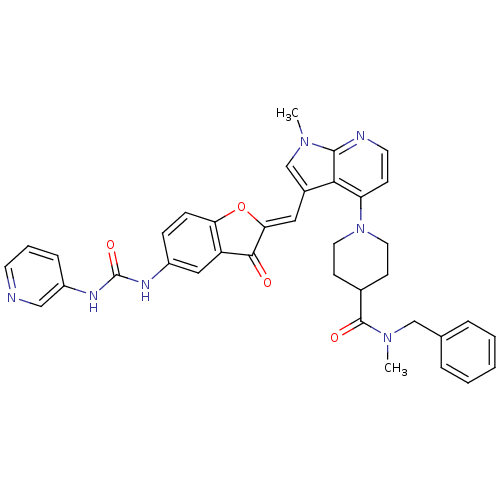
((Z)-N-benzyl-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-p...)Show SMILES CN(Cc1ccccc1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C37H35N7O4/c1-42-23-26(19-32-34(45)29-20-27(10-11-31(29)48-32)40-37(47)41-28-9-6-15-38-21-28)33-30(12-16-39-35(33)42)44-17-13-25(14-18-44)36(46)43(2)22-24-7-4-3-5-8-24/h3-12,15-16,19-21,23,25H,13-14,17-18,22H2,1-2H3,(H2,40,41,47)/b32-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314319
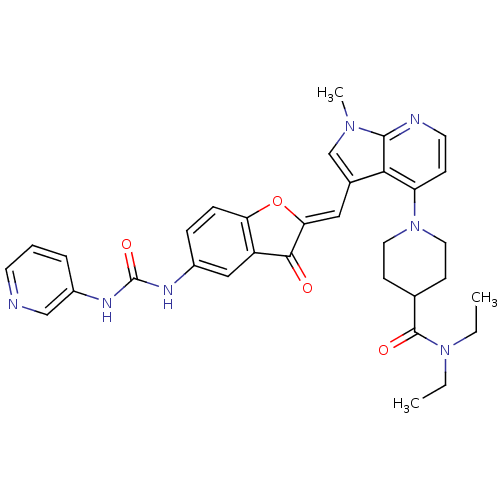
((Z)-N,N-diethyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin...)Show SMILES CCN(CC)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C33H35N7O4/c1-4-39(5-2)32(42)21-11-15-40(16-12-21)26-10-14-35-31-29(26)22(20-38(31)3)17-28-30(41)25-18-23(8-9-27(25)44-28)36-33(43)37-24-7-6-13-34-19-24/h6-10,13-14,17-21H,4-5,11-12,15-16H2,1-3H3,(H2,36,37,43)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314321

((Z)-1-(2-((4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1C2CCC1COC2 Show InChI InChI=1S/C29H26N6O4/c1-34-14-17(26-23(8-10-31-28(26)34)35-20-5-6-21(35)16-38-15-20)11-25-27(36)22-12-18(4-7-24(22)39-25)32-29(37)33-19-3-2-9-30-13-19/h2-4,7-14,20-21H,5-6,15-16H2,1H3,(H2,32,33,37)/b25-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314326
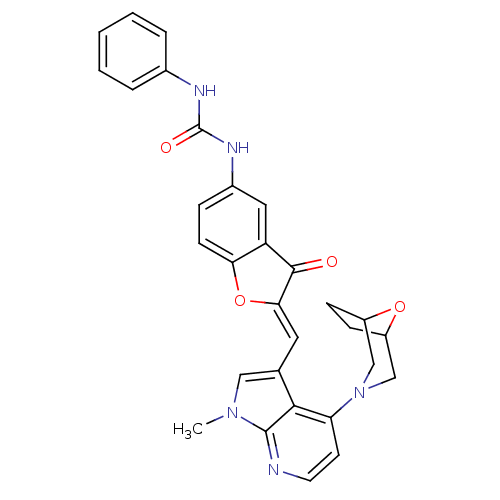
((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4ccccc4)cc3C2=O)c2c(ccnc12)N1CC2CCC(C1)O2 Show InChI InChI=1S/C30H27N5O4/c1-34-15-18(27-24(11-12-31-29(27)34)35-16-21-8-9-22(17-35)38-21)13-26-28(36)23-14-20(7-10-25(23)39-26)33-30(37)32-19-5-3-2-4-6-19/h2-7,10-15,21-22H,8-9,16-17H2,1H3,(H2,32,33,37)/b26-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314331

((Z)-1-methyl-3-(2-((1-methyl-4-phenyl-1H-pyrrolo[2...)Show SMILES CNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(-c5ccccc5)c34)C(=O)c2c1 Show InChI InChI=1S/C25H20N4O3/c1-26-25(31)28-17-8-9-20-19(13-17)23(30)21(32-20)12-16-14-29(2)24-22(16)18(10-11-27-24)15-6-4-3-5-7-15/h3-14H,1-2H3,(H2,26,28,31)/b21-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314322

((Z)-1-(2-((4-(2-oxa-5-azabicyclo[2.2.2]octan-5-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CC2CCC1CO2 Show InChI InChI=1S/C29H26N6O4/c1-34-14-17(26-23(8-10-31-28(26)34)35-15-21-6-5-20(35)16-38-21)11-25-27(36)22-12-18(4-7-24(22)39-25)32-29(37)33-19-3-2-9-30-13-19/h2-4,7-14,20-21H,5-6,15-16H2,1H3,(H2,32,33,37)/b25-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314323

((Z)-1-(2-((1-methyl-4-morpholino-1H-pyrrolo[2,3-b]...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCOCC1 Show InChI InChI=1S/C27H24N6O4/c1-32-16-17(24-21(6-8-29-26(24)32)33-9-11-36-12-10-33)13-23-25(34)20-14-18(4-5-22(20)37-23)30-27(35)31-19-3-2-7-28-15-19/h2-8,13-16H,9-12H2,1H3,(H2,30,31,35)/b23-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314319

((Z)-N,N-diethyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin...)Show SMILES CCN(CC)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C33H35N7O4/c1-4-39(5-2)32(42)21-11-15-40(16-12-21)26-10-14-35-31-29(26)22(20-38(31)3)17-28-30(41)25-18-23(8-9-27(25)44-28)36-33(43)37-24-7-6-13-34-19-24/h6-10,13-14,17-21H,4-5,11-12,15-16H2,1-3H3,(H2,36,37,43)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314328

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES CCNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(N5CC6CCC(C5)O6)c34)C(=O)c2c1 Show InChI InChI=1S/C26H27N5O4/c1-3-27-26(33)29-16-4-7-21-19(11-16)24(32)22(35-21)10-15-12-30(2)25-23(15)20(8-9-28-25)31-13-17-5-6-18(14-31)34-17/h4,7-12,17-18H,3,5-6,13-14H2,1-2H3,(H2,27,29,33)/b22-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314326

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4ccccc4)cc3C2=O)c2c(ccnc12)N1CC2CCC(C1)O2 Show InChI InChI=1S/C30H27N5O4/c1-34-15-18(27-24(11-12-31-29(27)34)35-16-21-8-9-22(17-35)38-21)13-26-28(36)23-14-20(7-10-25(23)39-26)33-30(37)32-19-5-3-2-4-6-19/h2-7,10-15,21-22H,8-9,16-17H2,1H3,(H2,32,33,37)/b26-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314329

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES CNC(=O)Nc1ccc2O\C(=C/c3cn(C)c4nccc(N5CC6CCC(C5)O6)c34)C(=O)c2c1 Show InChI InChI=1S/C25H25N5O4/c1-26-25(32)28-15-3-6-20-18(10-15)23(31)21(34-20)9-14-11-29(2)24-22(14)19(7-8-27-24)30-12-16-4-5-17(13-30)33-16/h3,6-11,16-17H,4-5,12-13H2,1-2H3,(H2,26,28,32)/b21-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314341

((Z)-1-(2-((1-methyl-4-(4-(piperidine-1-carbonyl)pi...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CCCCC1 Show InChI InChI=1S/C34H35N7O4/c1-39-21-23(18-29-31(42)26-19-24(7-8-28(26)45-29)37-34(44)38-25-6-5-12-35-20-25)30-27(9-13-36-32(30)39)40-16-10-22(11-17-40)33(43)41-14-3-2-4-15-41/h5-9,12-13,18-22H,2-4,10-11,14-17H2,1H3,(H2,37,38,44)/b29-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314342

((Z)-1-(2-((1-methyl-4-(4-(pyrrolidine-1-carbonyl)p...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C33H33N7O4/c1-38-20-22(29-26(8-12-35-31(29)38)39-15-9-21(10-16-39)32(42)40-13-2-3-14-40)17-28-30(41)25-18-23(6-7-27(25)44-28)36-33(43)37-24-5-4-11-34-19-24/h4-8,11-12,17-21H,2-3,9-10,13-16H2,1H3,(H2,36,37,43)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 909 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314337

((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(Cc1cccnc1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C36H34N8O4/c1-42-22-25(17-31-33(45)28-18-26(7-8-30(28)48-31)40-36(47)41-27-6-4-13-38-20-27)32-29(9-14-39-34(32)42)44-15-10-24(11-16-44)35(46)43(2)21-23-5-3-12-37-19-23/h3-9,12-14,17-20,22,24H,10-11,15-16,21H2,1-2H3,(H2,40,41,47)/b31-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314336

((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(Cc1ccncc1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C36H34N8O4/c1-42-22-25(18-31-33(45)28-19-26(5-6-30(28)48-31)40-36(47)41-27-4-3-12-38-20-27)32-29(9-15-39-34(32)42)44-16-10-24(11-17-44)35(46)43(2)21-23-7-13-37-14-8-23/h3-9,12-15,18-20,22,24H,10-11,16-17,21H2,1-2H3,(H2,40,41,47)/b31-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314335

((Z)-N-methyl-1-(1-methyl-3-((3-oxo-5-(3-pyridin-3-...)Show SMILES CN(Cc1ccccn1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C36H34N8O4/c1-42-21-24(18-31-33(45)28-19-25(8-9-30(28)48-31)40-36(47)41-26-7-5-13-37-20-26)32-29(10-15-39-34(32)42)44-16-11-23(12-17-44)35(46)43(2)22-27-6-3-4-14-38-27/h3-10,13-15,18-21,23H,11-12,16-17,22H2,1-2H3,(H2,40,41,47)/b31-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314316

((Z)-1-(2-((4-(4-(8-oxa-3-azabicyclo[3.2.1]octane-3...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CC2CCC(C1)O2 Show InChI InChI=1S/C35H35N7O5/c1-40-18-22(15-30-32(43)27-16-23(4-7-29(27)47-30)38-35(45)39-24-3-2-11-36-17-24)31-28(8-12-37-33(31)40)41-13-9-21(10-14-41)34(44)42-19-25-5-6-26(20-42)46-25/h2-4,7-8,11-12,15-18,21,25-26H,5-6,9-10,13-14,19-20H2,1H3,(H2,38,39,45)/b30-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314320

((Z)-N,N-dimethyl-1-(1-methyl-3-((3-oxo-5-(3-pyridi...)Show SMILES CN(C)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C31H31N7O4/c1-36(2)30(40)19-9-13-38(14-10-19)24-8-12-33-29-27(24)20(18-37(29)3)15-26-28(39)23-16-21(6-7-25(23)42-26)34-31(41)35-22-5-4-11-32-17-22/h4-8,11-12,15-19H,9-10,13-14H2,1-3H3,(H2,34,35,41)/b26-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314325

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CC2CCC(C1)O2 Show InChI InChI=1S/C29H26N6O4/c1-34-14-17(26-23(8-10-31-28(26)34)35-15-20-5-6-21(16-35)38-20)11-25-27(36)22-12-18(4-7-24(22)39-25)32-29(37)33-19-3-2-9-30-13-19/h2-4,7-14,20-21H,5-6,15-16H2,1H3,(H2,32,33,37)/b25-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314324

((Z)-1-(2-((4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4ccc(nc4)N4CCOCC4)cc3C2=O)c2c(ccnc12)N1CC2CCC(C1)O2 Show InChI InChI=1S/C33H33N7O5/c1-38-17-20(30-26(8-9-34-32(30)38)40-18-23-4-5-24(19-40)44-23)14-28-31(41)25-15-21(2-6-27(25)45-28)36-33(42)37-22-3-7-29(35-16-22)39-10-12-43-13-11-39/h2-3,6-9,14-17,23-24H,4-5,10-13,18-19H2,1H3,(H2,36,37,42)/b28-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314339

((Z)-1-(2-((4-(4-(3-methoxypyrrolidine-1-carbonyl)p...)Show SMILES COC1CCN(C1)C(=O)C1CCN(CC1)c1ccnc2n(C)cc(\C=C3/Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c12 Show InChI InChI=1S/C34H35N7O5/c1-39-19-22(16-29-31(42)26-17-23(5-6-28(26)46-29)37-34(44)38-24-4-3-11-35-18-24)30-27(7-12-36-32(30)39)40-13-8-21(9-14-40)33(43)41-15-10-25(20-41)45-2/h3-7,11-12,16-19,21,25H,8-10,13-15,20H2,1-2H3,(H2,37,38,44)/b29-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314318

((Z)-1-(2-((1-methyl-4-(4-(morpholine-4-carbonyl)pi...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1CCOCC1 Show InChI InChI=1S/C33H33N7O5/c1-38-20-22(17-28-30(41)25-18-23(4-5-27(25)45-28)36-33(43)37-24-3-2-9-34-19-24)29-26(6-10-35-31(29)38)39-11-7-21(8-12-39)32(42)40-13-15-44-16-14-40/h2-6,9-10,17-21H,7-8,11-16H2,1H3,(H2,36,37,43)/b28-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50314317

((Z)-1-(2-((4-(4-(3-oxa-8-azabicyclo[3.2.1]octane-8...)Show SMILES Cn1cc(\C=C2/Oc3ccc(NC(=O)Nc4cccnc4)cc3C2=O)c2c(ccnc12)N1CCC(CC1)C(=O)N1C2CCC1COC2 Show InChI InChI=1S/C35H35N7O5/c1-40-18-22(15-30-32(43)27-16-23(4-7-29(27)47-30)38-35(45)39-24-3-2-11-36-17-24)31-28(8-12-37-33(31)40)41-13-9-21(10-14-41)34(44)42-25-5-6-26(42)20-46-19-25/h2-4,7-8,11-12,15-18,21,25-26H,5-6,9-10,13-14,19-20H2,1H3,(H2,38,39,45)/b30-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50314330
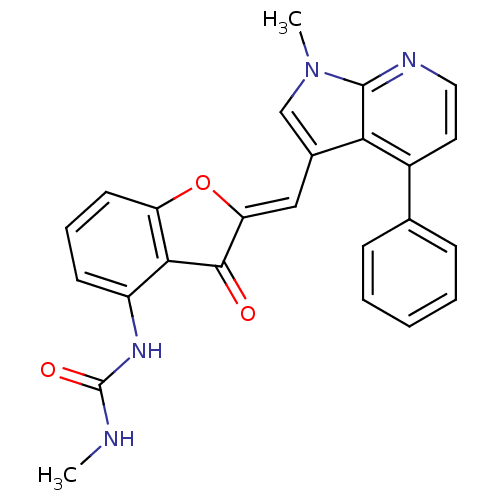
((Z)-1-methyl-3-(2-((1-methyl-4-phenyl-1H-pyrrolo[2...)Show SMILES CNC(=O)Nc1cccc2O\C(=C/c3cn(C)c4nccc(-c5ccccc5)c34)C(=O)c12 Show InChI InChI=1S/C25H20N4O3/c1-26-25(31)28-18-9-6-10-19-22(18)23(30)20(32-19)13-16-14-29(2)24-21(16)17(11-12-27-24)15-7-4-3-5-8-15/h3-14H,1-2H3,(H2,26,28,31)/b20-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 2259-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.012
BindingDB Entry DOI: 10.7270/Q2TB1717 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data