 Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50034099
Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50034099 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127832
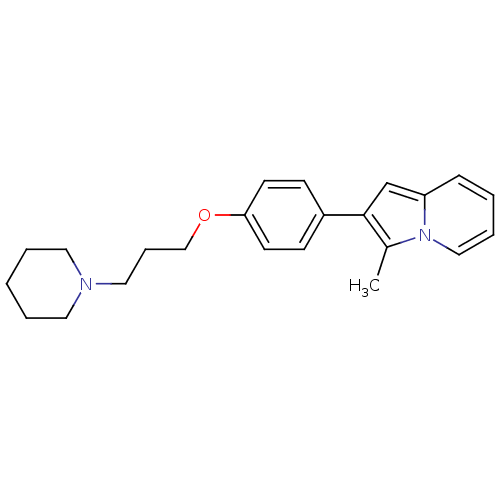
(3-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-23(18-21-8-3-6-16-25(19)21)20-9-11-22(12-10-20)26-17-7-15-24-13-4-2-5-14-24/h3,6,8-12,16,18H,2,4-5,7,13-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127844
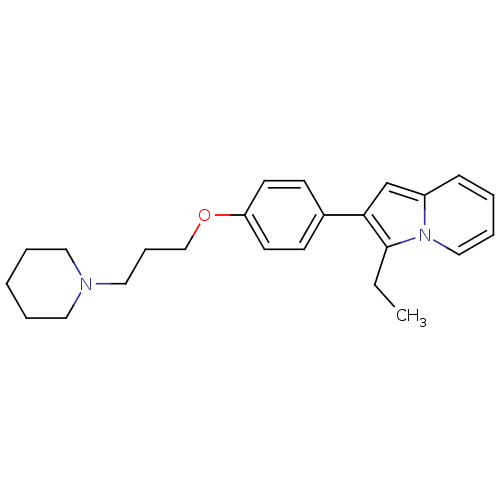
(3-Ethyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-in...)Show InChI InChI=1S/C24H30N2O/c1-2-24-23(19-21-9-4-7-17-26(21)24)20-10-12-22(13-11-20)27-18-8-16-25-14-5-3-6-15-25/h4,7,9-13,17,19H,2-3,5-6,8,14-16,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127838
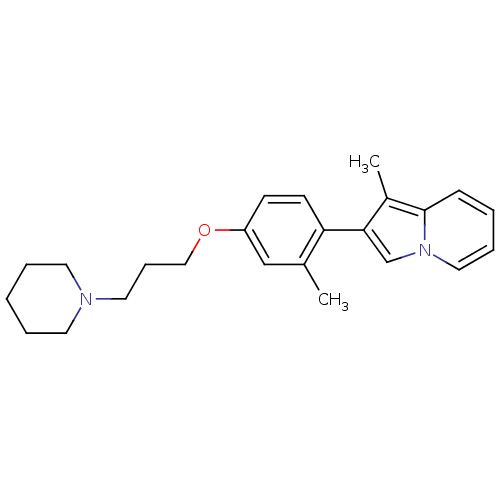
(1-Methyl-2-[2-methyl-4-(3-piperidin-1-yl-propoxy)-...)Show InChI InChI=1S/C24H30N2O/c1-19-17-21(27-16-8-14-25-12-5-3-6-13-25)10-11-22(19)23-18-26-15-7-4-9-24(26)20(23)2/h4,7,9-11,15,17-18H,3,5-6,8,12-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127841
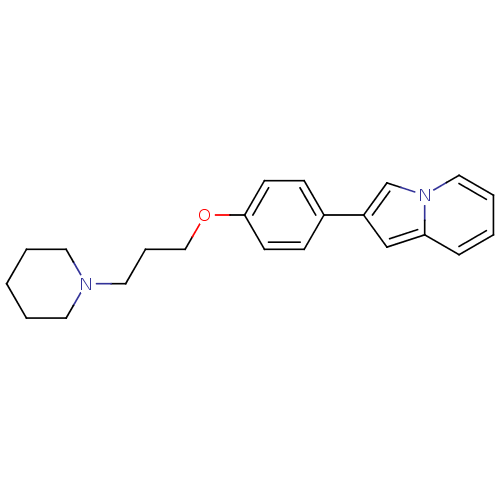
(2-[4-(3-Piperidin-1-yl-propoxy)-phenyl]-indolizine...)Show InChI InChI=1S/C22H26N2O/c1-3-12-23(13-4-1)14-6-16-25-22-10-8-19(9-11-22)20-17-21-7-2-5-15-24(21)18-20/h2,5,7-11,15,17-18H,1,3-4,6,12-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127834
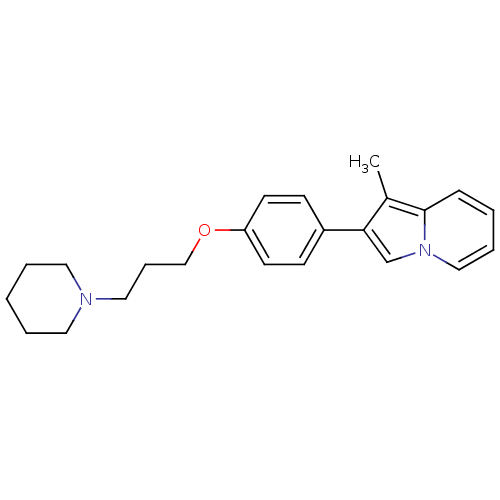
(1-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-22(18-25-16-6-3-8-23(19)25)20-9-11-21(12-10-20)26-17-7-15-24-13-4-2-5-14-24/h3,6,8-12,16,18H,2,4-5,7,13-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127840
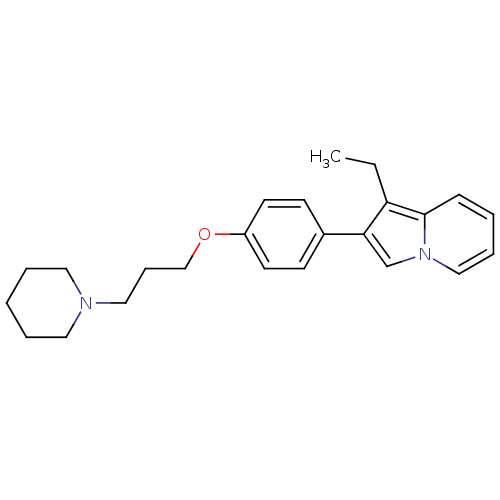
(1-Ethyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-in...)Show InChI InChI=1S/C24H30N2O/c1-2-22-23(19-26-17-7-4-9-24(22)26)20-10-12-21(13-11-20)27-18-8-16-25-14-5-3-6-15-25/h4,7,9-13,17,19H,2-3,5-6,8,14-16,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127830
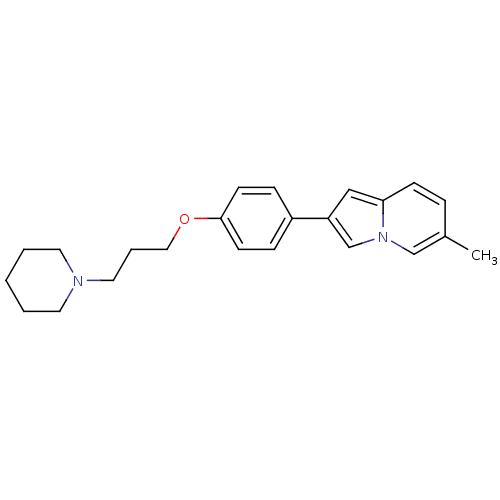
(6-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-6-9-22-16-21(18-25(22)17-19)20-7-10-23(11-8-20)26-15-5-14-24-12-3-2-4-13-24/h6-11,16-18H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127835
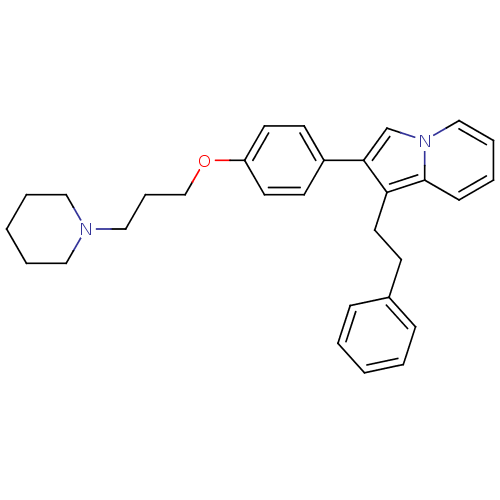
(1-Phenethyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl...)Show SMILES C(COc1ccc(cc1)-c1cn2ccccc2c1CCc1ccccc1)CN1CCCCC1 Show InChI InChI=1S/C30H34N2O/c1-3-10-25(11-4-1)13-18-28-29(24-32-22-8-5-12-30(28)32)26-14-16-27(17-15-26)33-23-9-21-31-19-6-2-7-20-31/h1,3-5,8,10-12,14-17,22,24H,2,6-7,9,13,18-21,23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127833
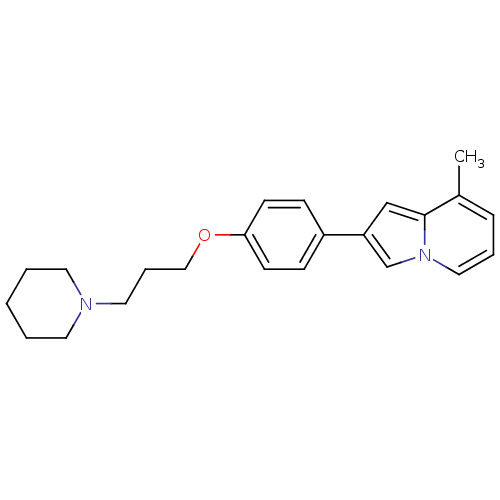
(8-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-7-5-15-25-18-21(17-23(19)25)20-8-10-22(11-9-20)26-16-6-14-24-12-3-2-4-13-24/h5,7-11,15,17-18H,2-4,6,12-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127847
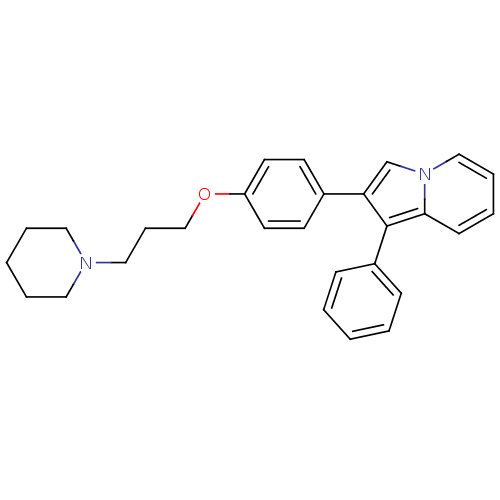
(1-Phenyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show SMILES C(COc1ccc(cc1)-c1cn2ccccc2c1-c1ccccc1)CN1CCCCC1 Show InChI InChI=1S/C28H30N2O/c1-3-10-24(11-4-1)28-26(22-30-20-8-5-12-27(28)30)23-13-15-25(16-14-23)31-21-9-19-29-17-6-2-7-18-29/h1,3-5,8,10-16,20,22H,2,6-7,9,17-19,21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127846
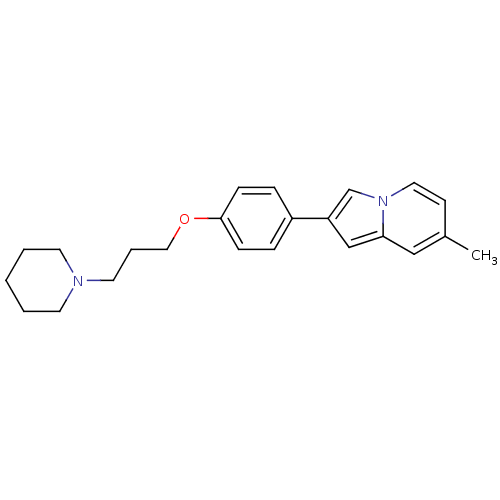
(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-10-14-25-18-21(17-22(25)16-19)20-6-8-23(9-7-20)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-18H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127843
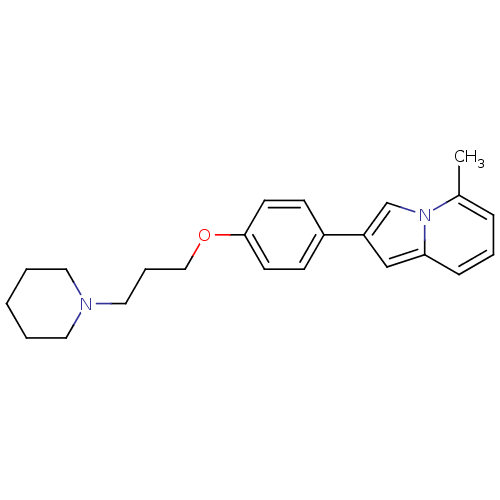
(5-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-7-5-8-22-17-21(18-25(19)22)20-9-11-23(12-10-20)26-16-6-15-24-13-3-2-4-14-24/h5,7-12,17-18H,2-4,6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127845
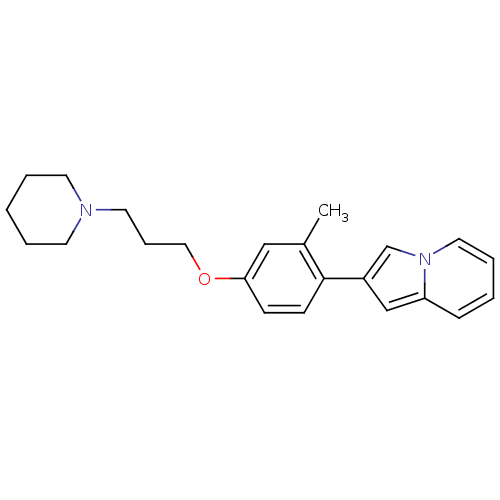
(2-[2-Methyl-4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C23H28N2O/c1-19-16-22(26-15-7-13-24-11-4-2-5-12-24)9-10-23(19)20-17-21-8-3-6-14-25(21)18-20/h3,6,8-10,14,16-18H,2,4-5,7,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127836
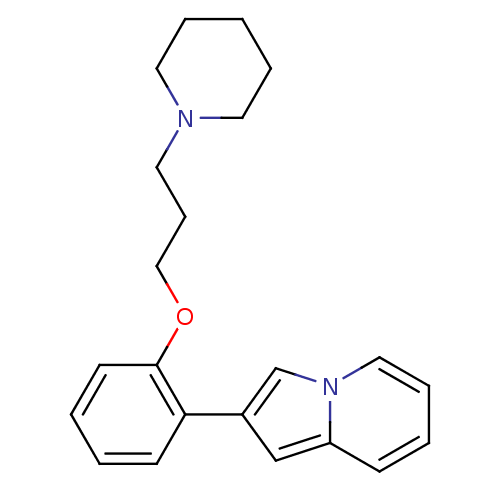
(2-[2-(3-Piperidin-1-yl-propoxy)-phenyl]-indolizine...)Show InChI InChI=1S/C22H26N2O/c1-5-12-23(13-6-1)14-8-16-25-22-11-3-2-10-21(22)19-17-20-9-4-7-15-24(20)18-19/h2-4,7,9-11,15,17-18H,1,5-6,8,12-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127842
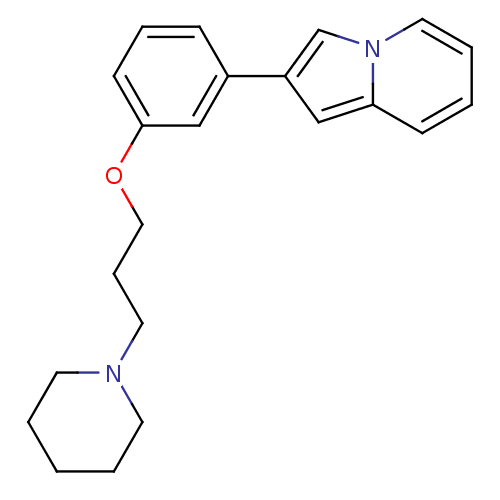
(2-[3-(3-Piperidin-1-yl-propoxy)-phenyl]-indolizine...)Show InChI InChI=1S/C22H26N2O/c1-3-11-23(12-4-1)13-7-15-25-22-10-6-8-19(17-22)20-16-21-9-2-5-14-24(21)18-20/h2,5-6,8-10,14,16-18H,1,3-4,7,11-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50357014
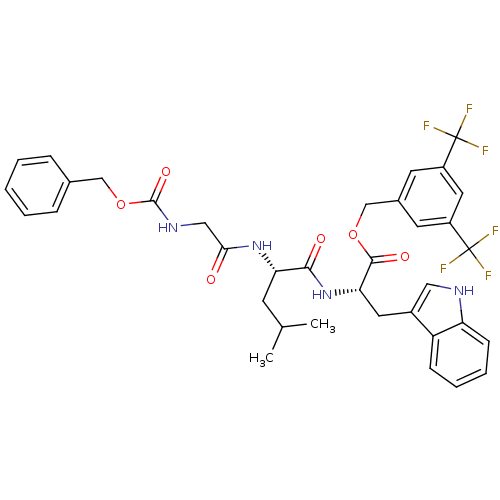
(CHEMBL1916636)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C36H36F6N4O6/c1-21(2)12-29(45-31(47)18-44-34(50)52-19-22-8-4-3-5-9-22)32(48)46-30(15-24-17-43-28-11-7-6-10-27(24)28)33(49)51-20-23-13-25(35(37,38)39)16-26(14-23)36(40,41)42/h3-11,13-14,16-17,21,29-30,43H,12,15,18-20H2,1-2H3,(H,44,50)(H,45,47)(H,46,48)/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50357011
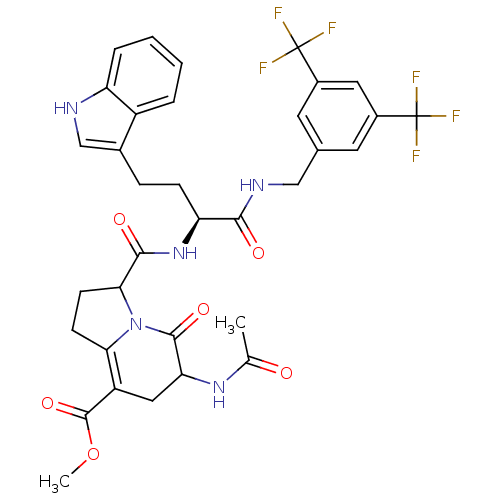
(CHEMBL1916633)Show SMILES COC(=O)C1=C2CCC(N2C(=O)C(C1)NC(C)=O)C(=O)N[C@@H](CCc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:4| Show InChI InChI=1S/C34H33F6N5O6/c1-17(46)43-26-14-23(32(50)51-2)27-9-10-28(45(27)31(26)49)30(48)44-25(8-7-19-16-41-24-6-4-3-5-22(19)24)29(47)42-15-18-11-20(33(35,36)37)13-21(12-18)34(38,39)40/h3-6,11-13,16,25-26,28,41H,7-10,14-15H2,1-2H3,(H,42,47)(H,43,46)(H,44,48)/t25-,26?,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50357013
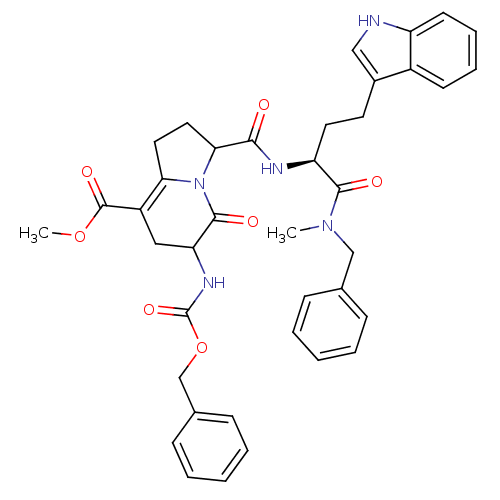
(CHEMBL1916635)Show SMILES COC(=O)C1=C2CCC(N2C(=O)C(C1)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1c[nH]c2ccccc12)C(=O)N(C)Cc1ccccc1 |r,c:4| Show InChI InChI=1S/C39H41N5O7/c1-43(23-25-11-5-3-6-12-25)36(46)31(18-17-27-22-40-30-16-10-9-15-28(27)30)41-35(45)34-20-19-33-29(38(48)50-2)21-32(37(47)44(33)34)42-39(49)51-24-26-13-7-4-8-14-26/h3-16,22,31-32,34,40H,17-21,23-24H2,1-2H3,(H,41,45)(H,42,49)/t31-,32?,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50357013

(CHEMBL1916635)Show SMILES COC(=O)C1=C2CCC(N2C(=O)C(C1)NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1c[nH]c2ccccc12)C(=O)N(C)Cc1ccccc1 |r,c:4| Show InChI InChI=1S/C39H41N5O7/c1-43(23-25-11-5-3-6-12-25)36(46)31(18-17-27-22-40-30-16-10-9-15-28(27)30)41-35(45)34-20-19-33-29(38(48)50-2)21-32(37(47)44(33)34)42-39(49)51-24-26-13-7-4-8-14-26/h3-16,22,31-32,34,40H,17-21,23-24H2,1-2H3,(H,41,45)(H,42,49)/t31-,32?,34?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50357012
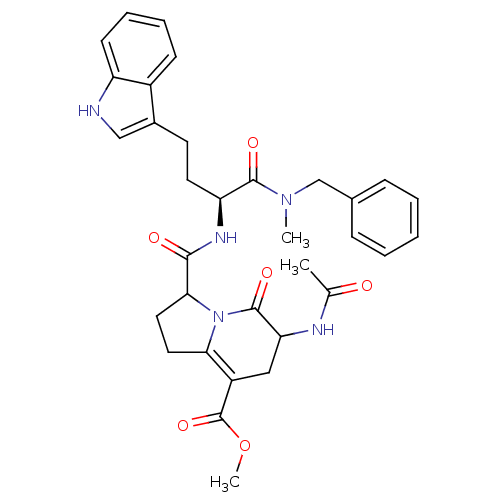
(CHEMBL1916634)Show SMILES COC(=O)C1=C2CCC(N2C(=O)C(C1)NC(C)=O)C(=O)N[C@@H](CCc1c[nH]c2ccccc12)C(=O)N(C)Cc1ccccc1 |r,c:4| Show InChI InChI=1S/C33H37N5O6/c1-20(39)35-27-17-24(33(43)44-3)28-15-16-29(38(28)32(27)42)30(40)36-26(31(41)37(2)19-21-9-5-4-6-10-21)14-13-22-18-34-25-12-8-7-11-23(22)25/h4-12,18,26-27,29,34H,13-17,19H2,1-3H3,(H,35,39)(H,36,40)/t26-,27?,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50357014

(CHEMBL1916636)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C36H36F6N4O6/c1-21(2)12-29(45-31(47)18-44-34(50)52-19-22-8-4-3-5-9-22)32(48)46-30(15-24-17-43-28-11-7-6-10-27(24)28)33(49)51-20-23-13-25(35(37,38)39)16-26(14-23)36(40,41)42/h3-11,13-14,16-17,21,29-30,43H,12,15,18-20H2,1-2H3,(H,44,50)(H,45,47)(H,46,48)/t29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50357012

(CHEMBL1916634)Show SMILES COC(=O)C1=C2CCC(N2C(=O)C(C1)NC(C)=O)C(=O)N[C@@H](CCc1c[nH]c2ccccc12)C(=O)N(C)Cc1ccccc1 |r,c:4| Show InChI InChI=1S/C33H37N5O6/c1-20(39)35-27-17-24(33(43)44-3)28-15-16-29(38(28)32(27)42)30(40)36-26(31(41)37(2)19-21-9-5-4-6-10-21)14-13-22-18-34-25-12-8-7-11-23(22)25/h4-12,18,26-27,29,34H,13-17,19H2,1-3H3,(H,35,39)(H,36,40)/t26-,27?,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50357011

(CHEMBL1916633)Show SMILES COC(=O)C1=C2CCC(N2C(=O)C(C1)NC(C)=O)C(=O)N[C@@H](CCc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:4| Show InChI InChI=1S/C34H33F6N5O6/c1-17(46)43-26-14-23(32(50)51-2)27-9-10-28(45(27)31(26)49)30(48)44-25(8-7-19-16-41-24-6-4-3-5-22(19)24)29(47)42-15-18-11-20(33(35,36)37)13-21(12-18)34(38,39)40/h3-6,11-13,16,25-26,28,41H,7-10,14-15H2,1-2H3,(H,42,47)(H,43,46)(H,44,48)/t25-,26?,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human NK2 receptor expressed in CHO cells |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50085657
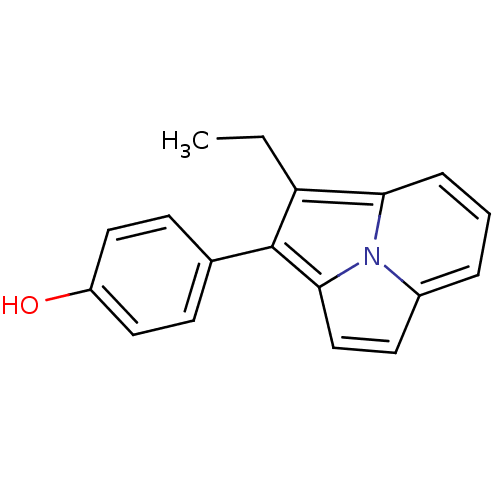
(4-(1-Ethyl-pyrrolo[2,1,5-cd]indolizin-2-yl)-phenol...)Show InChI InChI=1S/C18H15NO/c1-2-15-16-5-3-4-13-8-11-17(19(13)16)18(15)12-6-9-14(20)10-7-12/h3-11,20H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Agonist activity at estrogen receptor |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50318405
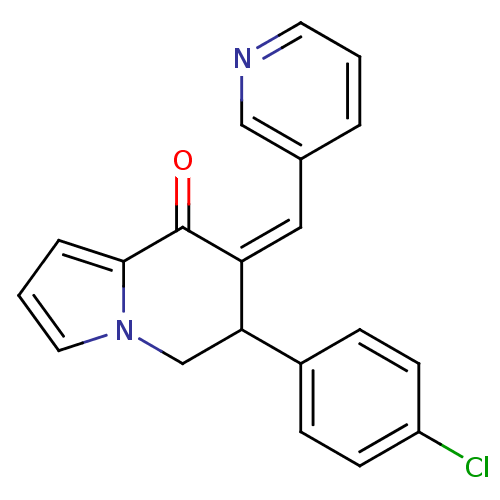
((Z)-6-(4-chlorophenyl)-7-(pyridin-3-ylmethylene)-6...)Show InChI InChI=1S/C20H15ClN2O/c21-16-7-5-15(6-8-16)18-13-23-10-2-4-19(23)20(24)17(18)11-14-3-1-9-22-12-14/h1-12,18H,13H2/b17-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50070097
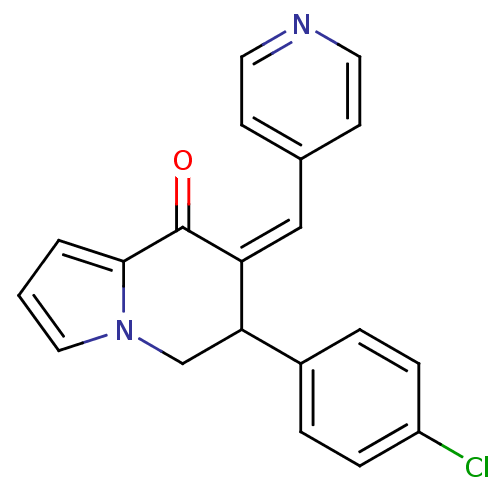
((Z)-6-(4-chlorophenyl)-7-(pyridin-4-ylmethylene)-6...)Show InChI InChI=1S/C20H15ClN2O/c21-16-5-3-15(4-6-16)18-13-23-11-1-2-19(23)20(24)17(18)12-14-7-9-22-10-8-14/h1-12,18H,13H2/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357002
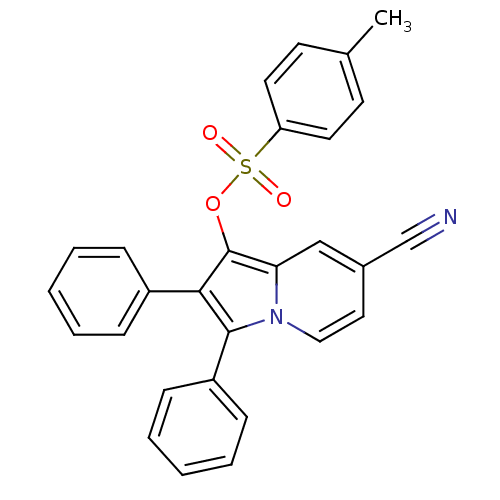
(CHEMBL1916429)Show SMILES Cc1ccc(cc1)S(=O)(=O)Oc1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1 Show InChI InChI=1S/C28H20N2O3S/c1-20-12-14-24(15-13-20)34(31,32)33-28-25-18-21(19-29)16-17-30(25)27(23-10-6-3-7-11-23)26(28)22-8-4-2-5-9-22/h2-18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357009
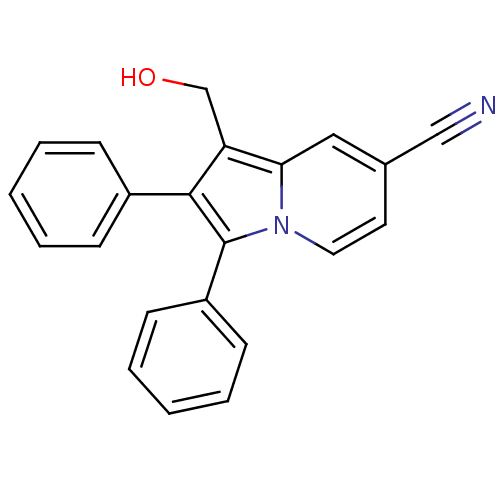
(CHEMBL1916445)Show InChI InChI=1S/C22H16N2O/c23-14-16-11-12-24-20(13-16)19(15-25)21(17-7-3-1-4-8-17)22(24)18-9-5-2-6-10-18/h1-13,25H,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357005
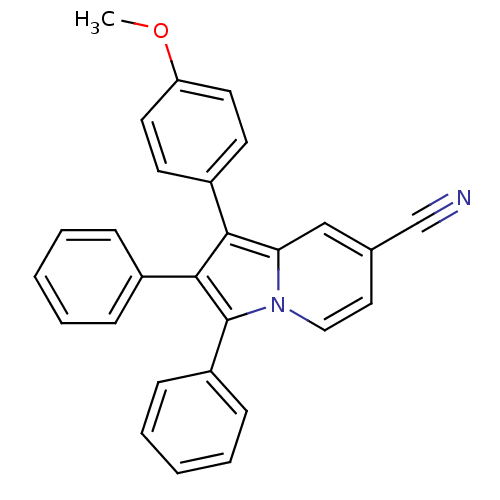
(CHEMBL1916437)Show SMILES COc1ccc(cc1)-c1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1 Show InChI InChI=1S/C28H20N2O/c1-31-24-14-12-22(13-15-24)26-25-18-20(19-29)16-17-30(25)28(23-10-6-3-7-11-23)27(26)21-8-4-2-5-9-21/h2-18H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357000
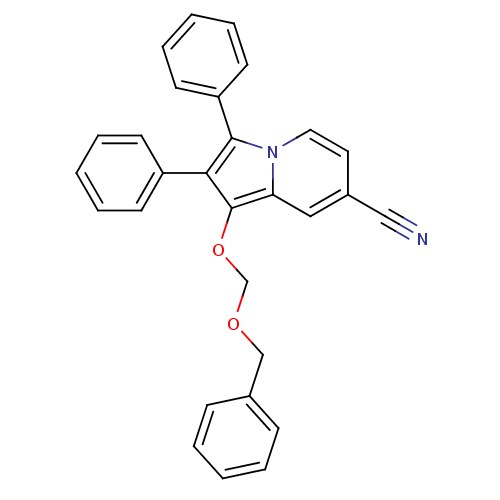
(CHEMBL1916426)Show SMILES N#Cc1ccn2c(c(c(OCOCc3ccccc3)c2c1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C29H22N2O2/c30-19-23-16-17-31-26(18-23)29(33-21-32-20-22-10-4-1-5-11-22)27(24-12-6-2-7-13-24)28(31)25-14-8-3-9-15-25/h1-18H,20-21H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357008
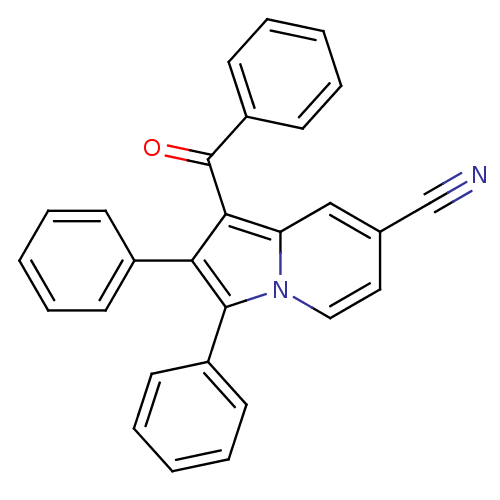
(CHEMBL1916444)Show SMILES O=C(c1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H18N2O/c29-19-20-16-17-30-24(18-20)26(28(31)23-14-8-3-9-15-23)25(21-10-4-1-5-11-21)27(30)22-12-6-2-7-13-22/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357007
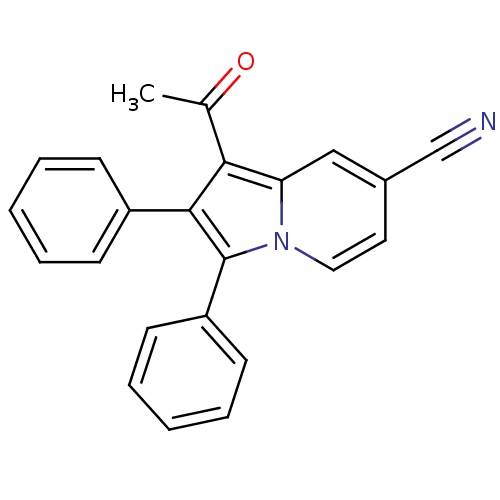
(CHEMBL1914479)Show SMILES CC(=O)c1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1 Show InChI InChI=1S/C23H16N2O/c1-16(26)21-20-14-17(15-24)12-13-25(20)23(19-10-6-3-7-11-19)22(21)18-8-4-2-5-9-18/h2-14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357004
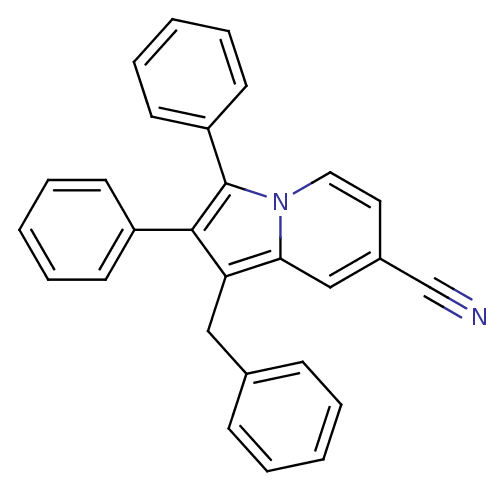
(CHEMBL1916435)Show SMILES N#Cc1ccn2c(c(c(Cc3ccccc3)c2c1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C28H20N2/c29-20-22-16-17-30-26(19-22)25(18-21-10-4-1-5-11-21)27(23-12-6-2-7-13-23)28(30)24-14-8-3-9-15-24/h1-17,19H,18H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357010
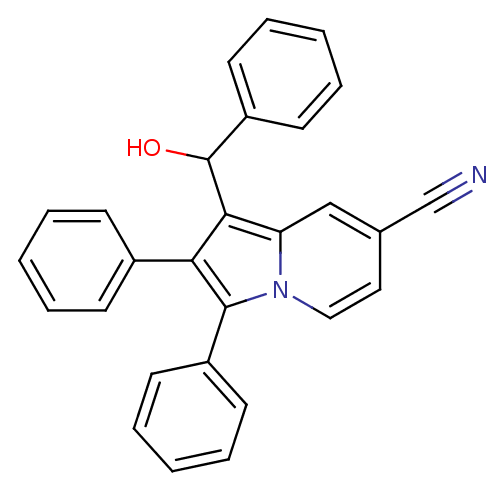
(CHEMBL1916447)Show SMILES OC(c1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H20N2O/c29-19-20-16-17-30-24(18-20)26(28(31)23-14-8-3-9-15-23)25(21-10-4-1-5-11-21)27(30)22-12-6-2-7-13-22/h1-18,28,31H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357016
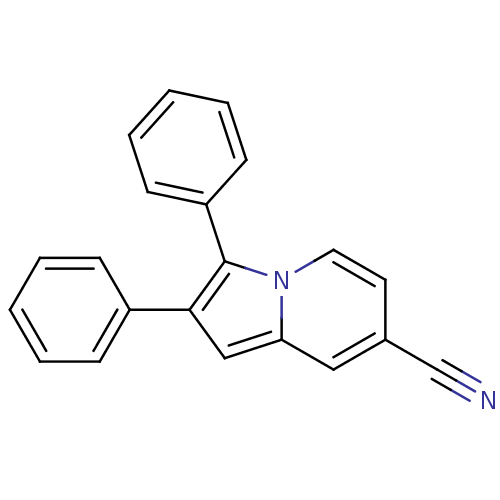
(CHEMBL1916417)Show InChI InChI=1S/C21H14N2/c22-15-16-11-12-23-19(13-16)14-20(17-7-3-1-4-8-17)21(23)18-9-5-2-6-10-18/h1-14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357001
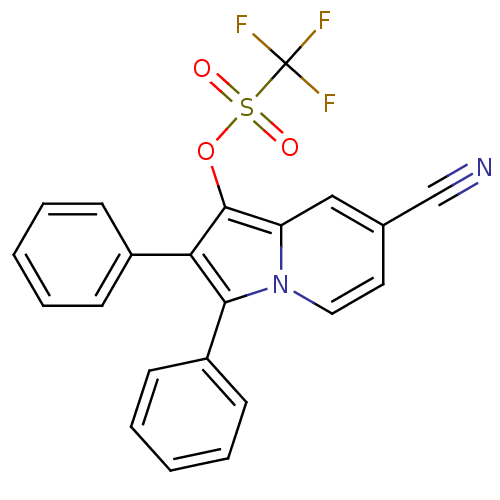
(CHEMBL1916428)Show SMILES FC(F)(F)S(=O)(=O)Oc1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1 Show InChI InChI=1S/C22H13F3N2O3S/c23-22(24,25)31(28,29)30-21-18-13-15(14-26)11-12-27(18)20(17-9-5-2-6-10-17)19(21)16-7-3-1-4-8-16/h1-13H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357006
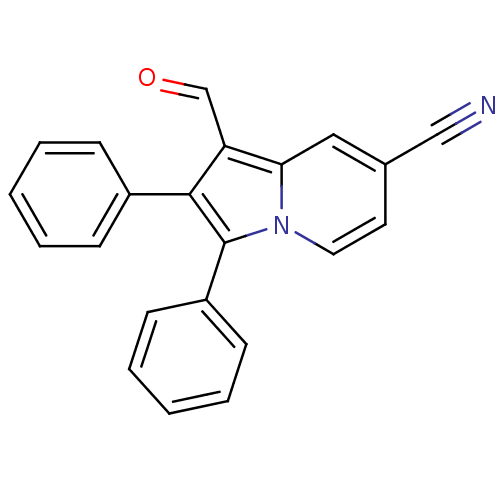
(CHEMBL1916443)Show SMILES O=Cc1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1 Show InChI InChI=1S/C22H14N2O/c23-14-16-11-12-24-20(13-16)19(15-25)21(17-7-3-1-4-8-17)22(24)18-9-5-2-6-10-18/h1-13,15H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357003
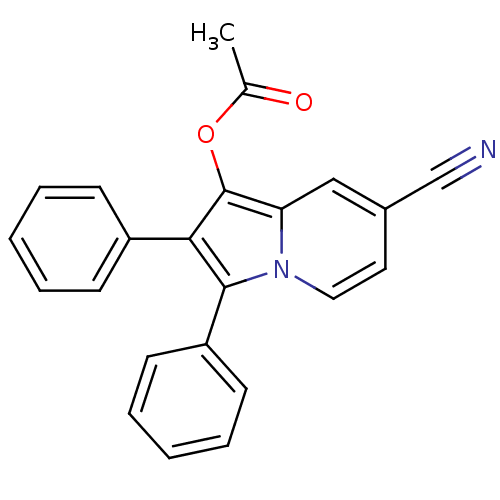
(CHEMBL1916430)Show SMILES CC(=O)Oc1c(c(-c2ccccc2)n2ccc(cc12)C#N)-c1ccccc1 Show InChI InChI=1S/C23H16N2O2/c1-16(26)27-23-20-14-17(15-24)12-13-25(20)22(19-10-6-3-7-11-19)21(23)18-8-4-2-5-9-18/h2-14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50357015
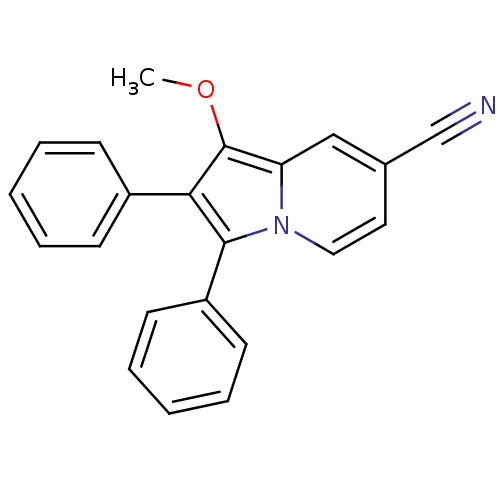
(CHEMBL1916418)Show InChI InChI=1S/C22H16N2O/c1-25-22-19-14-16(15-23)12-13-24(19)21(18-10-6-3-7-11-18)20(22)17-8-4-2-5-9-17/h2-14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana
Curated by ChEMBL
| Assay Description
Inhibition of rabbit reticulocytes arachidonate 15-lipoxygenase |
Eur J Med Chem 46: 5237-57 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.042
BindingDB Entry DOI: 10.7270/Q2ZG6SNF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data