 Found 67 hits Enz. Inhib. hit(s) with all data for entry = 50036686
Found 67 hits Enz. Inhib. hit(s) with all data for entry = 50036686 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
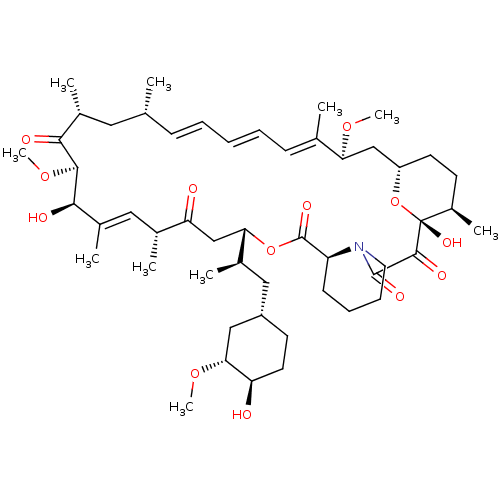
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50030448
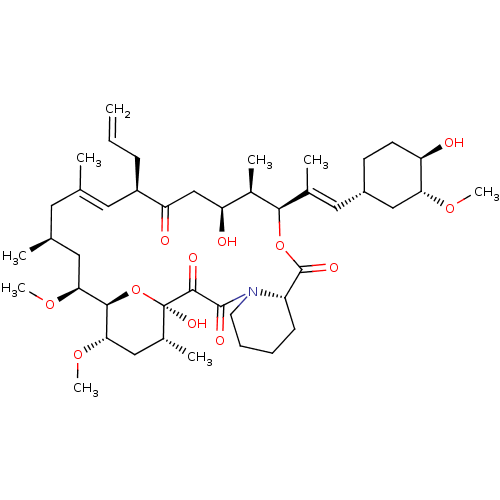
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068608
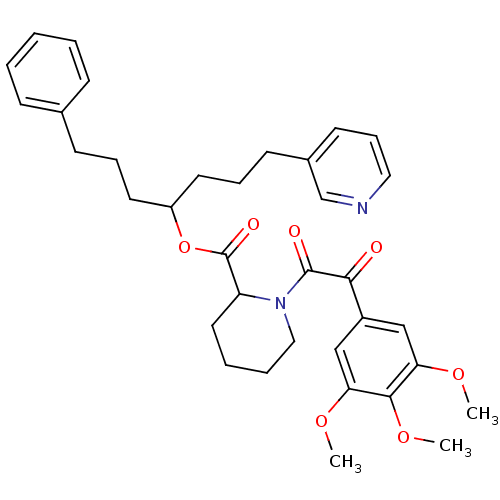
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 Show InChI InChI=1S/C35H42N2O7/c1-41-30-22-27(23-31(42-2)33(30)43-3)32(38)34(39)37-21-8-7-19-29(37)35(40)44-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-36-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408684
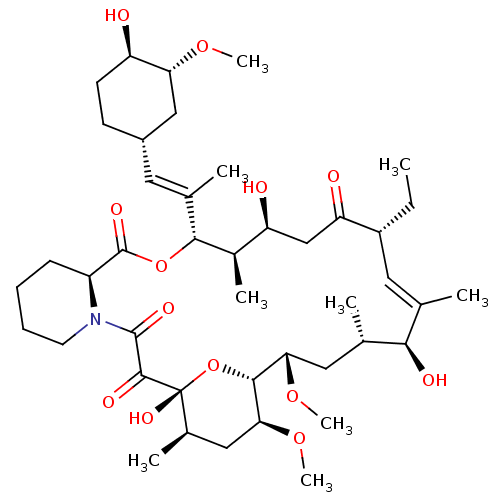
(CHEMBL2052020 | L-685818)Show SMILES CC[C@@H]1\C=C(C)/[C@@H](O)[C@@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |t:3| Show InChI InChI=1S/C43H69NO13/c1-10-29-18-23(2)37(48)24(3)19-35(54-8)39-36(55-9)20-26(5)43(52,57-39)40(49)41(50)44-16-12-11-13-30(44)42(51)56-38(27(6)32(46)22-33(29)47)25(4)17-28-14-15-31(45)34(21-28)53-7/h17-18,24,26-32,34-39,45-46,48,52H,10-16,19-22H2,1-9H3/b23-18-,25-17+/t24-,26+,27+,28-,29+,30-,31+,32-,34+,35-,36-,37+,38+,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068556
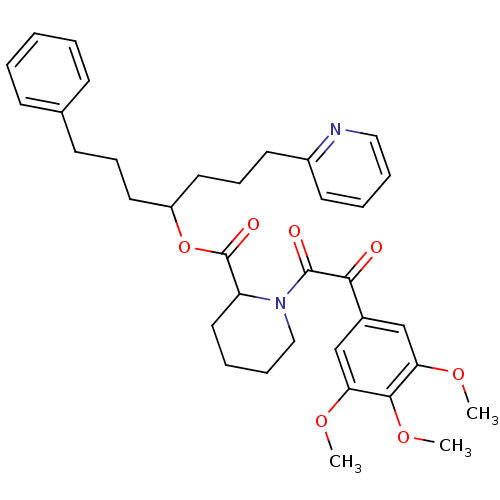
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccn1 Show InChI InChI=1S/C35H42N2O7/c1-41-30-23-26(24-31(42-2)33(30)43-3)32(38)34(39)37-22-10-8-20-29(37)35(40)44-28(18-11-15-25-13-5-4-6-14-25)19-12-17-27-16-7-9-21-36-27/h4-7,9,13-14,16,21,23-24,28-29H,8,10-12,15,17-20,22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068597
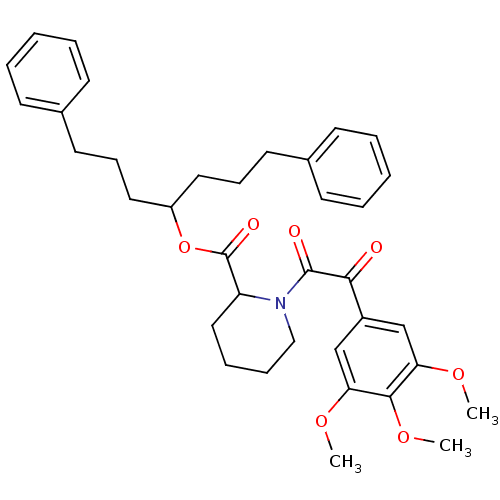
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C36H43NO7/c1-41-31-24-28(25-32(42-2)34(31)43-3)33(38)35(39)37-23-11-10-22-30(37)36(40)44-29(20-12-18-26-14-6-4-7-15-26)21-13-19-27-16-8-5-9-17-27/h4-9,14-17,24-25,29-30H,10-13,18-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068564
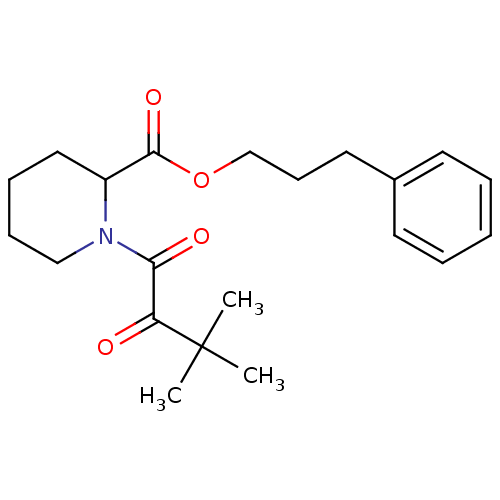
(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1ccccc1 Show InChI InChI=1S/C21H29NO4/c1-21(2,3)18(23)19(24)22-14-8-7-13-17(22)20(25)26-15-9-12-16-10-5-4-6-11-16/h4-6,10-11,17H,7-9,12-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068573
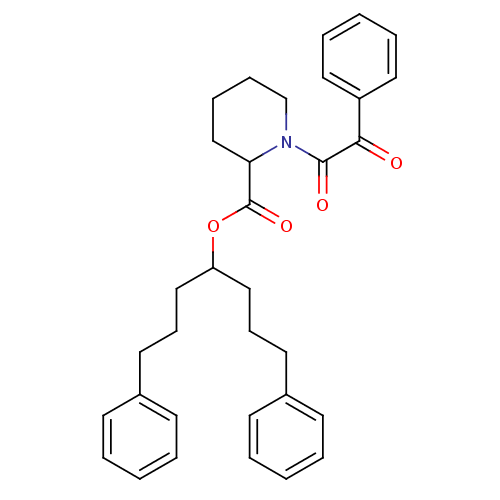
(1-(2-Oxo-2-phenyl-acetyl)-piperidine-2-carboxylic ...)Show SMILES O=C(OC(CCCc1ccccc1)CCCc1ccccc1)C1CCCCN1C(=O)C(=O)c1ccccc1 Show InChI InChI=1S/C33H37NO4/c35-31(28-20-8-3-9-21-28)32(36)34-25-11-10-24-30(34)33(37)38-29(22-12-18-26-14-4-1-5-15-26)23-13-19-27-16-6-2-7-17-27/h1-9,14-17,20-21,29-30H,10-13,18-19,22-25H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068578
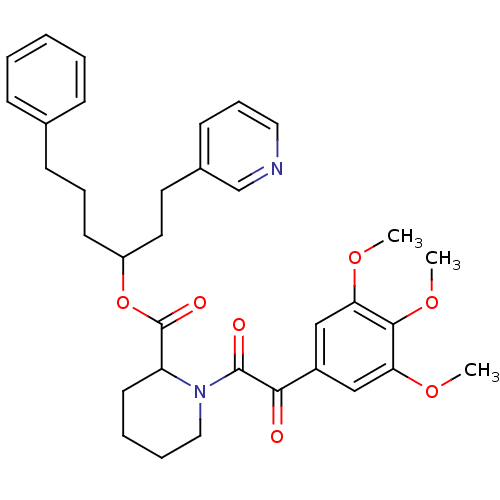
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCc1cccnc1 Show InChI InChI=1S/C34H40N2O7/c1-40-29-21-26(22-30(41-2)32(29)42-3)31(37)33(38)36-20-8-7-16-28(36)34(39)43-27(18-17-25-14-10-19-35-23-25)15-9-13-24-11-5-4-6-12-24/h4-6,10-12,14,19,21-23,27-28H,7-9,13,15-18,20H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068590

(1-(3,3-Dimethyl-2-oxo-butyryl)-pyrrolidine-2-carbo...)Show InChI InChI=1S/C19H26N2O4/c1-19(2,3)16(22)17(23)21-11-5-9-15(21)18(24)25-12-6-8-14-7-4-10-20-13-14/h4,7,10,13,15H,5-6,8-9,11-12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068582
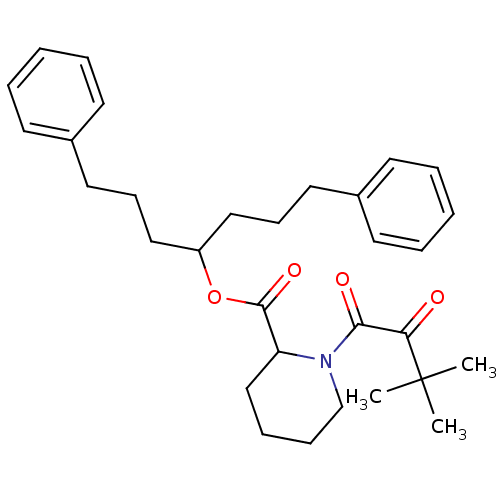
(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C31H41NO4/c1-31(2,3)28(33)29(34)32-23-11-10-22-27(32)30(35)36-26(20-12-18-24-14-6-4-7-15-24)21-13-19-25-16-8-5-9-17-25/h4-9,14-17,26-27H,10-13,18-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068547
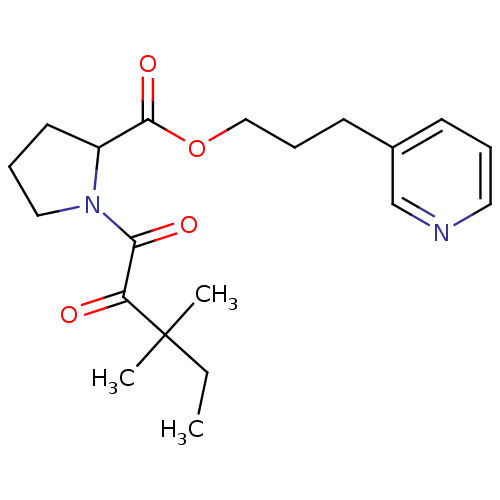
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC1C(=O)OCCCc1cccnc1 Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068610

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-19-12-11-17-24(29)27(32)33-20-18-23(21-13-7-5-8-14-21)22-15-9-6-10-16-22/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068576
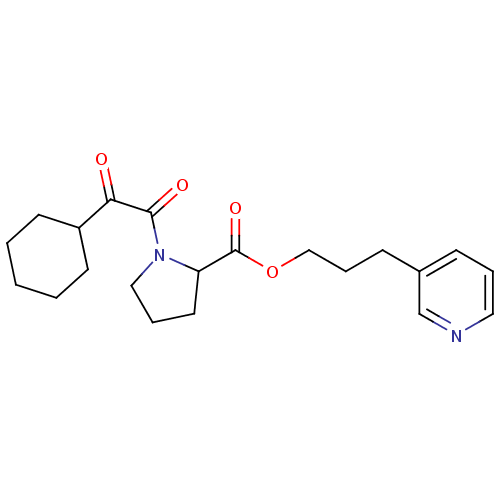
(1-(2-Cyclohexyl-2-oxo-acetyl)-pyrrolidine-2-carbox...)Show InChI InChI=1S/C21H28N2O4/c24-19(17-9-2-1-3-10-17)20(25)23-13-5-11-18(23)21(26)27-14-6-8-16-7-4-12-22-15-16/h4,7,12,15,17-18H,1-3,5-6,8-11,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068601
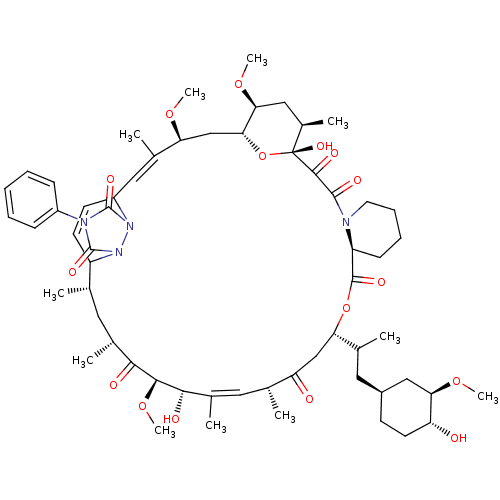
(CHEMBL265972 | WAY-124466)Show SMILES CO[C@@H]1C[C@H](CC(C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)C3C=CC(\C=C(C)\[C@H](C[C@H]4O[C@](O)([C@H](C)C[C@@H]4OC)C(=O)C(=O)N4CCCC[C@H]4C(=O)O2)OC)n2n3c(=O)n(-c3ccccc3)c2=O)CC[C@H]1O |c:14,30,33| Show InChI InChI=1S/C60H86N4O16/c1-33-25-37(5)52(67)54(78-11)53(68)38(6)26-34(2)46(66)31-48(35(3)27-40-20-23-45(65)49(30-40)76-9)79-57(71)44-19-15-16-24-61(44)56(70)55(69)60(74)39(7)29-50(77-10)51(80-60)32-47(75-8)36(4)28-42-21-22-43(33)64-59(73)62(58(72)63(42)64)41-17-13-12-14-18-41/h12-14,17-18,21-22,26,28,33-35,37,39-40,42-45,47-51,53-54,65,68,74H,15-16,19-20,23-25,27,29-32H2,1-11H3/b36-28+,38-26+/t33-,34+,35?,37+,39+,40-,42?,43?,44-,45+,47-,48-,49+,50-,51+,53-,54-,60+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068586
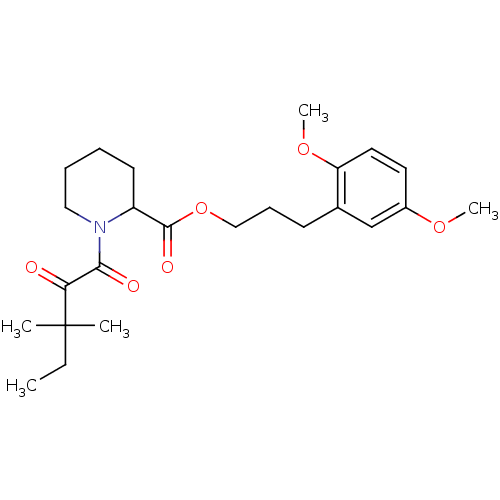
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1cc(OC)ccc1OC Show InChI InChI=1S/C24H35NO6/c1-6-24(2,3)21(26)22(27)25-14-8-7-11-19(25)23(28)31-15-9-10-17-16-18(29-4)12-13-20(17)30-5/h12-13,16,19H,6-11,14-15H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068545

(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1ccc(CCCCOC(=O)C2CCCCN2C(=O)C(=O)c2cc(OC)c(OC)c(OC)c2)cc1 Show InChI InChI=1S/C28H35NO8/c1-33-21-13-11-19(12-14-21)9-6-8-16-37-28(32)22-10-5-7-15-29(22)27(31)25(30)20-17-23(34-2)26(36-4)24(18-20)35-3/h11-14,17-18,22H,5-10,15-16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068553
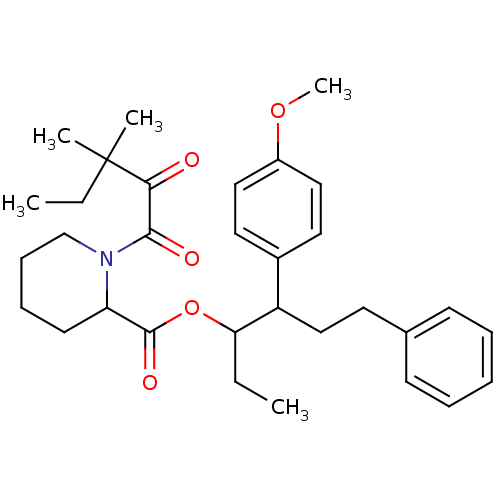
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(OC(=O)C1CCCCN1C(=O)C(=O)C(C)(C)CC)C(CCc1ccccc1)c1ccc(OC)cc1 Show InChI InChI=1S/C32H43NO5/c1-6-28(26(21-16-23-13-9-8-10-14-23)24-17-19-25(37-5)20-18-24)38-31(36)27-15-11-12-22-33(27)30(35)29(34)32(3,4)7-2/h8-10,13-14,17-20,26-28H,6-7,11-12,15-16,21-22H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068605
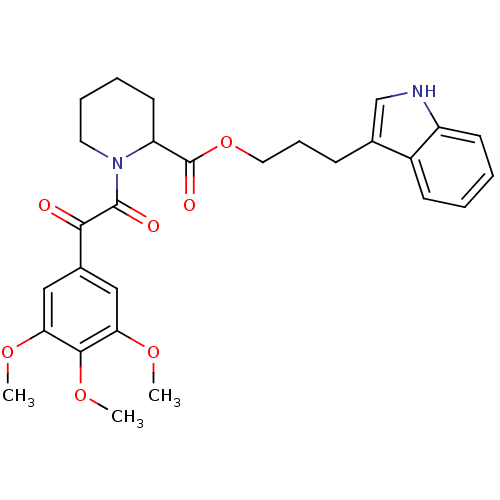
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C28H32N2O7/c1-34-23-15-19(16-24(35-2)26(23)36-3)25(31)27(32)30-13-7-6-12-22(30)28(33)37-14-8-9-18-17-29-21-11-5-4-10-20(18)21/h4-5,10-11,15-17,22,29H,6-9,12-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068577

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-20-12-11-17-23(29)27(32)33-24(22-15-9-6-10-16-22)19-18-21-13-7-5-8-14-21/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t23?,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068568
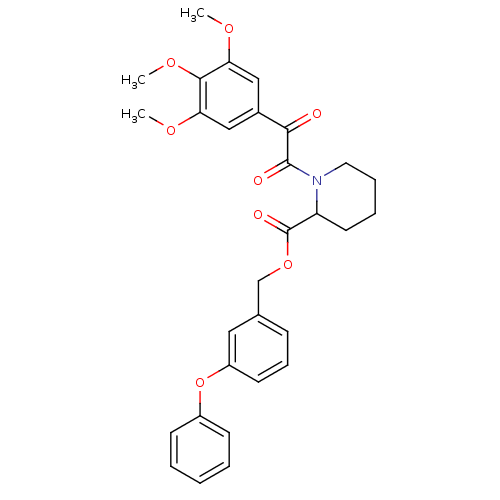
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OCc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C30H31NO8/c1-35-25-17-21(18-26(36-2)28(25)37-3)27(32)29(33)31-15-8-7-14-24(31)30(34)38-19-20-10-9-13-23(16-20)39-22-11-5-4-6-12-22/h4-6,9-13,16-18,24H,7-8,14-15,19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068594
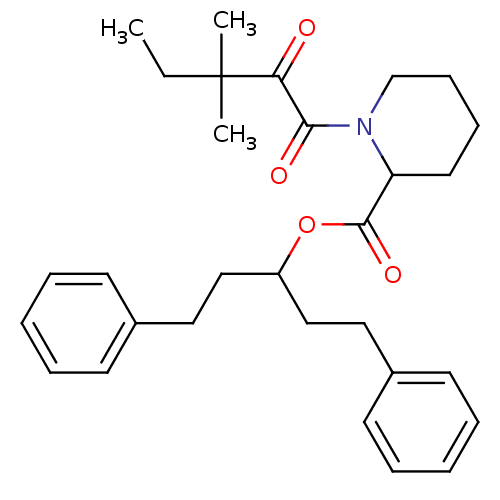
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OC(CCc1ccccc1)CCc1ccccc1 Show InChI InChI=1S/C30H39NO4/c1-4-30(2,3)27(32)28(33)31-22-12-11-17-26(31)29(34)35-25(20-18-23-13-7-5-8-14-23)21-19-24-15-9-6-10-16-24/h5-10,13-16,25-26H,4,11-12,17-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068591

(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES COc1ccc(CCCCOC(=O)C2CCCCN2C(=O)C(=O)C(C)(C)C)cc1 Show InChI InChI=1S/C23H33NO5/c1-23(2,3)20(25)21(26)24-15-7-5-10-19(24)22(27)29-16-8-6-9-17-11-13-18(28-4)14-12-17/h11-14,19H,5-10,15-16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068589

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C25H37NO7/c1-7-25(2,3)22(27)23(28)26-13-9-8-12-18(26)24(29)33-14-10-11-17-15-19(30-4)21(32-6)20(16-17)31-5/h15-16,18H,7-14H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068572
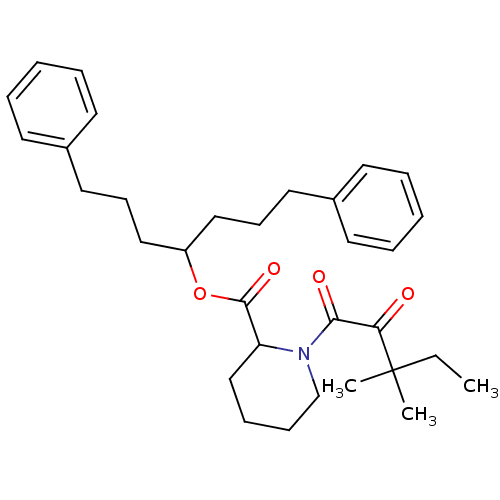
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C32H43NO4/c1-4-32(2,3)29(34)30(35)33-24-12-11-23-28(33)31(36)37-27(21-13-19-25-15-7-5-8-16-25)22-14-20-26-17-9-6-10-18-26/h5-10,15-18,27-28H,4,11-14,19-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068592

(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCC1CCCCC1 Show InChI InChI=1S/C21H35NO4/c1-21(2,3)18(23)19(24)22-14-8-7-13-17(22)20(25)26-15-9-12-16-10-5-4-6-11-16/h16-17H,4-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068581
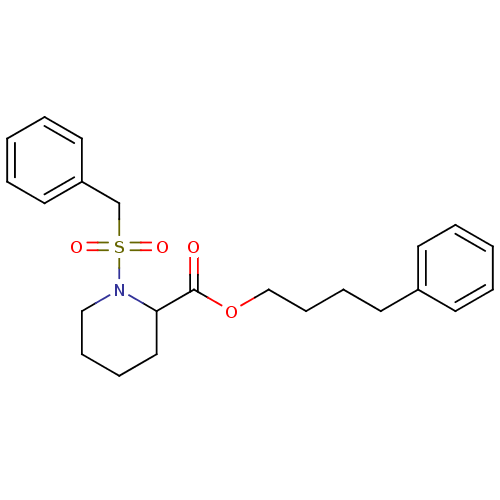
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES O=C(OCCCCc1ccccc1)C1CCCCN1S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H29NO4S/c25-23(28-18-10-8-13-20-11-3-1-4-12-20)22-16-7-9-17-24(22)29(26,27)19-21-14-5-2-6-15-21/h1-6,11-12,14-15,22H,7-10,13,16-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068599
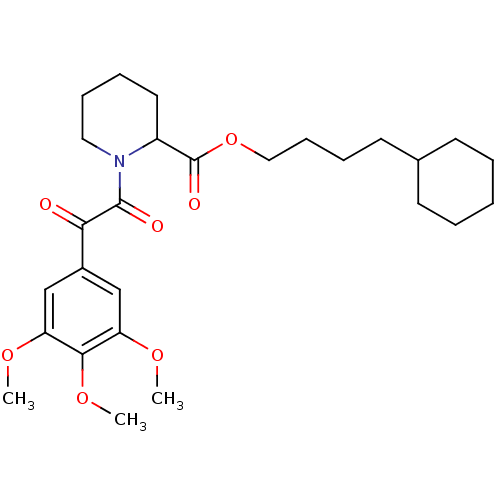
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OCCCCC1CCCCC1 Show InChI InChI=1S/C27H39NO7/c1-32-22-17-20(18-23(33-2)25(22)34-3)24(29)26(30)28-15-9-7-14-21(28)27(31)35-16-10-8-13-19-11-5-4-6-12-19/h17-19,21H,4-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068559
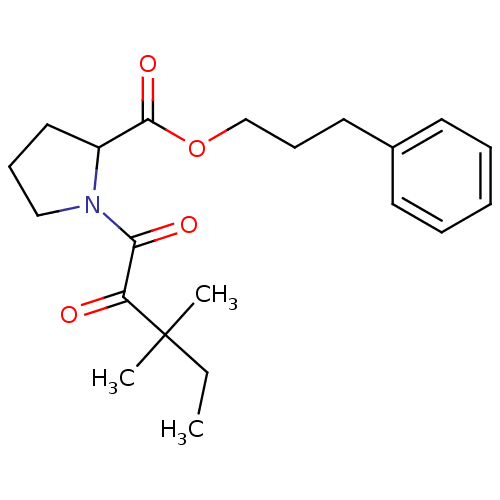
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC1C(=O)OCCCc1ccccc1 Show InChI InChI=1S/C21H29NO4/c1-4-21(2,3)18(23)19(24)22-14-8-13-17(22)20(25)26-15-9-12-16-10-6-5-7-11-16/h5-7,10-11,17H,4,8-9,12-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068558
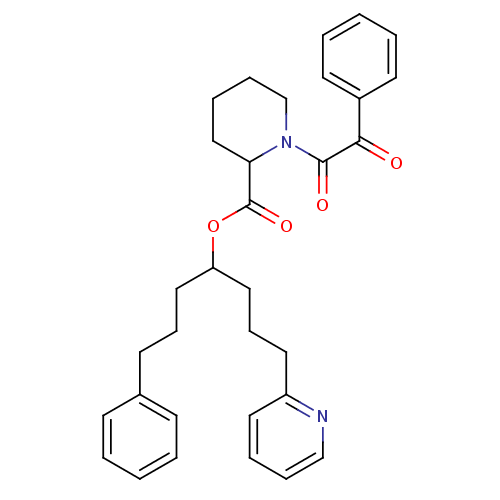
(1-(2-Oxo-2-phenyl-acetyl)-piperidine-2-carboxylic ...)Show SMILES O=C(OC(CCCc1ccccc1)CCCc1ccccn1)C1CCCCN1C(=O)C(=O)c1ccccc1 Show InChI InChI=1S/C32H36N2O4/c35-30(26-16-5-2-6-17-26)31(36)34-24-10-8-22-29(34)32(37)38-28(20-11-15-25-13-3-1-4-14-25)21-12-19-27-18-7-9-23-33-27/h1-7,9,13-14,16-18,23,28-29H,8,10-12,15,19-22,24H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068569
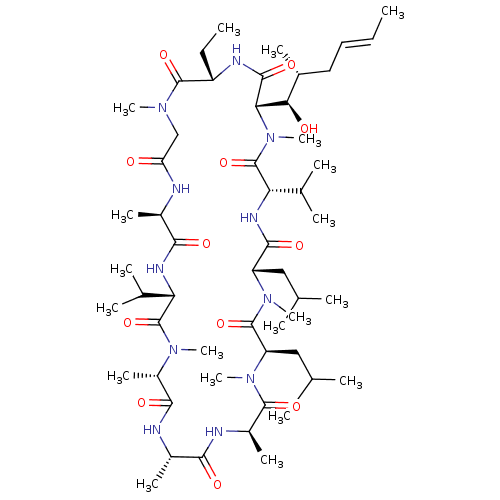
(27-Ethyl-30-(1-hydroxy-2-methyl-hex-4-enyl)-3,6-di...)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](C)N(C)C(=O)[C@@H](NC(=O)[C@@H](C)NC(=O)CN(C)C(=O)[C@H](CC)NC1=O)C(C)C)C(C)C Show InChI InChI=1S/C54H95N11O12/c1-21-23-24-32(11)44(67)43-49(72)58-37(22-2)51(74)61(16)27-40(66)55-33(12)46(69)59-41(30(7)8)53(76)62(17)36(15)47(70)56-34(13)45(68)57-35(14)50(73)64(19)39(26-29(5)6)52(75)63(18)38(25-28(3)4)48(71)60-42(31(9)10)54(77)65(43)20/h21,23,28-39,41-44,67H,22,24-27H2,1-20H3,(H,55,66)(H,56,70)(H,57,68)(H,58,72)(H,59,69)(H,60,71)/b23-21+/t32-,33-,34+,35-,36+,37+,38+,39-,41+,42+,43+,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068596

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCCc1ccc(OC)cc1 Show InChI InChI=1S/C24H35NO5/c1-5-24(2,3)21(26)22(27)25-16-8-6-11-20(25)23(28)30-17-9-7-10-18-12-14-19(29-4)15-13-18/h12-15,20H,5-11,16-17H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068607
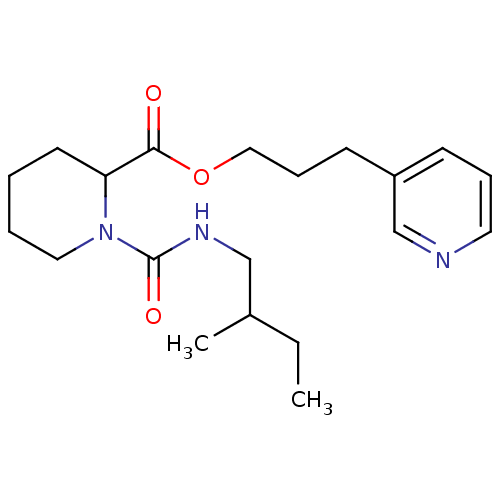
(1-(2-Methyl-butylcarbamoyl)-piperidine-2-carboxyli...)Show InChI InChI=1S/C20H31N3O3/c1-3-16(2)14-22-20(25)23-12-5-4-10-18(23)19(24)26-13-7-9-17-8-6-11-21-15-17/h6,8,11,15-16,18H,3-5,7,9-10,12-14H2,1-2H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068551
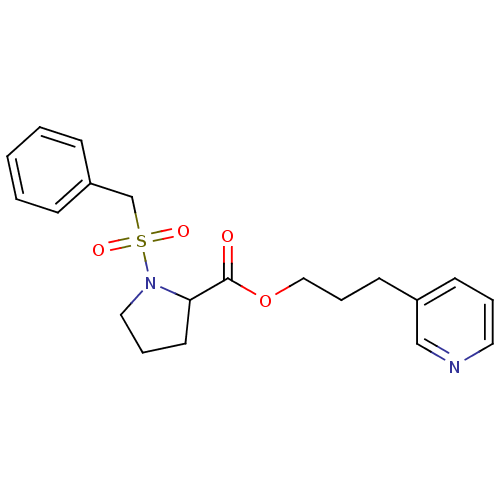
(1-Phenylmethanesulfonyl-pyrrolidine-2-carboxylic a...)Show InChI InChI=1S/C20H24N2O4S/c23-20(26-14-6-10-17-9-4-12-21-15-17)19-11-5-13-22(19)27(24,25)16-18-7-2-1-3-8-18/h1-4,7-9,12,15,19H,5-6,10-11,13-14,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068584
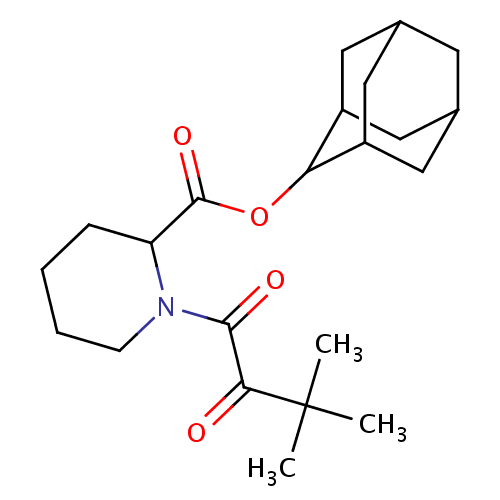
(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OC1C2CC3CC(C2)CC1C3 |TLB:26:25:23:20.19.21,THB:21:20:17:22.23.24,21:22:17:20.19.26,16:17:23:20.19.21,26:20:23:17.25.24| Show InChI InChI=1S/C22H33NO4/c1-22(2,3)19(24)20(25)23-7-5-4-6-17(23)21(26)27-18-15-9-13-8-14(11-15)12-16(18)10-13/h13-18H,4-12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
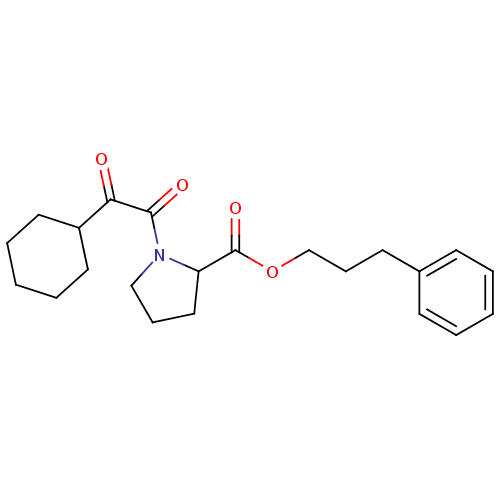
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068587
(1-(2-Cyclohexyl-2-oxo-acetyl)-pyrrolidine-2-carbox...)Show InChI InChI=1S/C22H29NO4/c24-20(18-12-5-2-6-13-18)21(25)23-15-7-14-19(23)22(26)27-16-8-11-17-9-3-1-4-10-17/h1,3-4,9-10,18-19H,2,5-8,11-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068562
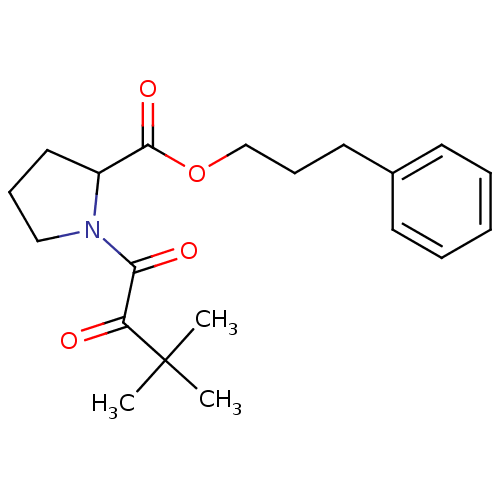
(1-(3,3-Dimethyl-2-oxo-butyryl)-pyrrolidine-2-carbo...)Show InChI InChI=1S/C20H27NO4/c1-20(2,3)17(22)18(23)21-13-7-12-16(21)19(24)25-14-8-11-15-9-5-4-6-10-15/h4-6,9-10,16H,7-8,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068609

(1-(2-Cyclohexyl-2-oxo-acetyl)-piperidine-2-carboxy...)Show SMILES COc1ccc(CCCCOC(=O)C2CCCCN2C(=O)C(=O)C2CCCCC2)cc1 Show InChI InChI=1S/C25H35NO5/c1-30-21-15-13-19(14-16-21)9-6-8-18-31-25(29)22-12-5-7-17-26(22)24(28)23(27)20-10-3-2-4-11-20/h13-16,20,22H,2-12,17-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068560
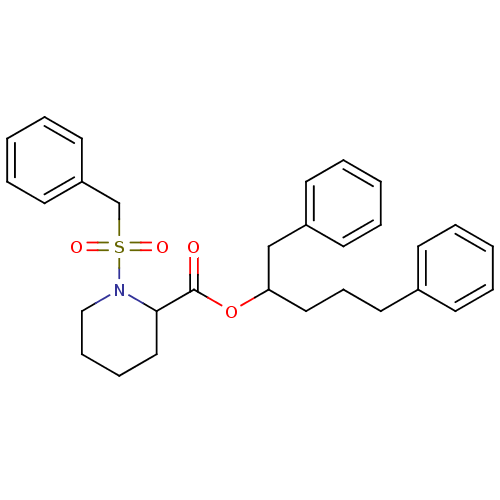
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES O=C(OC(CCCc1ccccc1)Cc1ccccc1)C1CCCCN1S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C30H35NO4S/c32-30(29-21-10-11-22-31(29)36(33,34)24-27-17-8-3-9-18-27)35-28(23-26-15-6-2-7-16-26)20-12-19-25-13-4-1-5-14-25/h1-9,13-18,28-29H,10-12,19-24H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068552
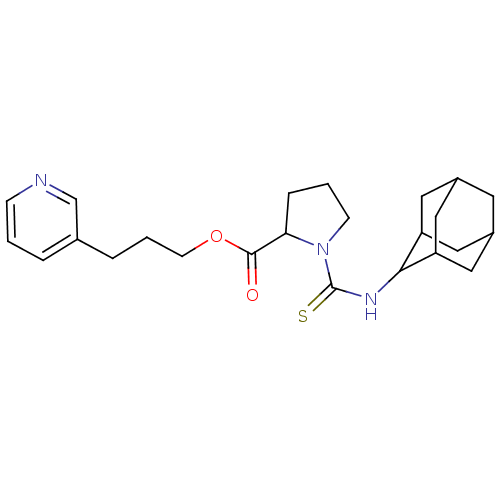
(1-(Adamantan-2-ylthiocarbamoyl)-pyrrolidine-2-carb...)Show SMILES O=C(OCCCc1cccnc1)C1CCCN1C(=S)NC1C2CC3CC(C2)CC1C3 |TLB:29:28:26:23.22.24,THB:24:23:20:25.26.27,24:25:20:23.22.29,19:20:26:23.22.24,29:23:26:20.28.27,(9.21,-4.9,;8.89,-3.39,;10.04,-2.38,;11.51,-2.87,;12.65,-1.84,;14.12,-2.33,;15.26,-1.31,;16.73,-1.8,;17.87,-.78,;17.57,.74,;16.1,1.21,;14.96,.2,;7.45,-2.92,;6.84,-1.35,;5.18,-1.43,;4.74,-3.06,;6.14,-3.96,;6.23,-5.64,;7.56,-6.42,;4.91,-6.4,;4.9,-7.94,;3.55,-8.68,;3.55,-10.22,;4.87,-11.01,;3.1,-11.32,;3.11,-9.78,;2.01,-8.68,;4.44,-9,;6.23,-8.71,;6.21,-10.25,)| Show InChI InChI=1S/C24H33N3O2S/c28-23(29-9-3-5-16-4-1-7-25-15-16)21-6-2-8-27(21)24(30)26-22-19-11-17-10-18(13-19)14-20(22)12-17/h1,4,7,15,17-22H,2-3,5-6,8-14H2,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068563
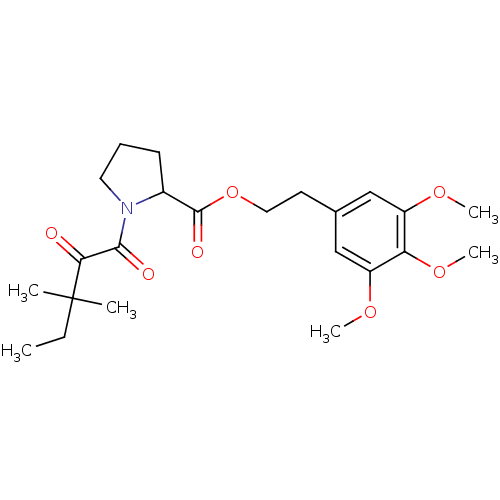
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC1C(=O)OCCc1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C23H33NO7/c1-7-23(2,3)20(25)21(26)24-11-8-9-16(24)22(27)31-12-10-15-13-17(28-4)19(30-6)18(14-15)29-5/h13-14,16H,7-12H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068588
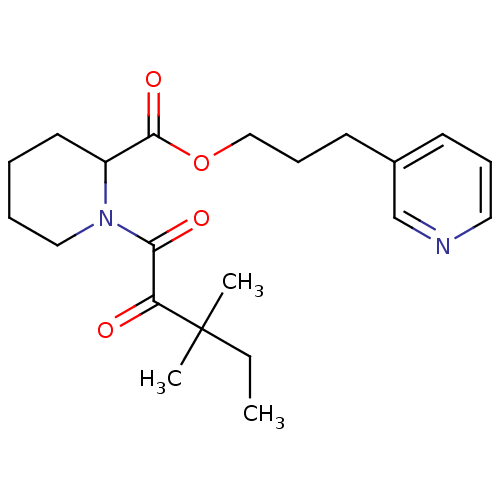
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1cccnc1 Show InChI InChI=1S/C21H30N2O4/c1-4-21(2,3)18(24)19(25)23-13-6-5-11-17(23)20(26)27-14-8-10-16-9-7-12-22-15-16/h7,9,12,15,17H,4-6,8,10-11,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068606
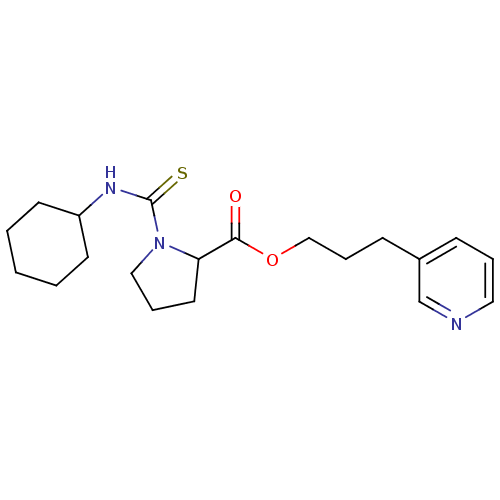
(1-Cyclohexylthiocarbamoyl-pyrrolidine-2-carboxylic...)Show InChI InChI=1S/C20H29N3O2S/c24-19(25-14-6-8-16-7-4-12-21-15-16)18-11-5-13-23(18)20(26)22-17-9-2-1-3-10-17/h4,7,12,15,17-18H,1-3,5-6,8-11,13-14H2,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068585
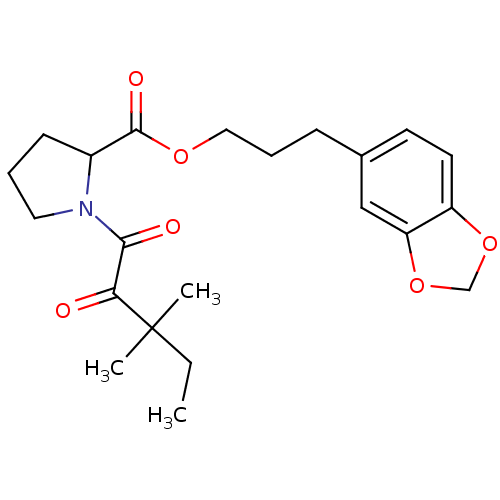
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC1C(=O)OCCCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H29NO6/c1-4-22(2,3)19(24)20(25)23-11-5-8-16(23)21(26)27-12-6-7-15-9-10-17-18(13-15)29-14-28-17/h9-10,13,16H,4-8,11-12,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068550
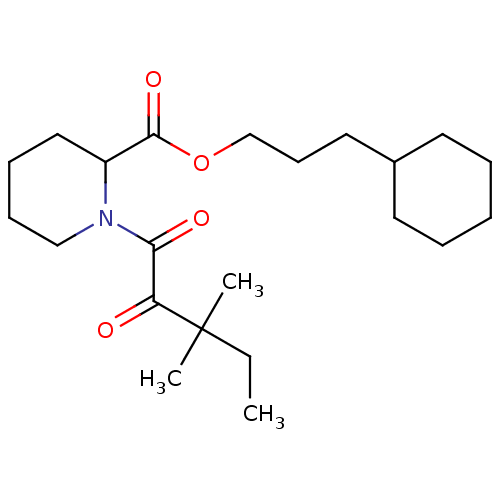
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCC1CCCCC1 Show InChI InChI=1S/C22H37NO4/c1-4-22(2,3)19(24)20(25)23-15-9-8-14-18(23)21(26)27-16-10-13-17-11-6-5-7-12-17/h17-18H,4-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068566
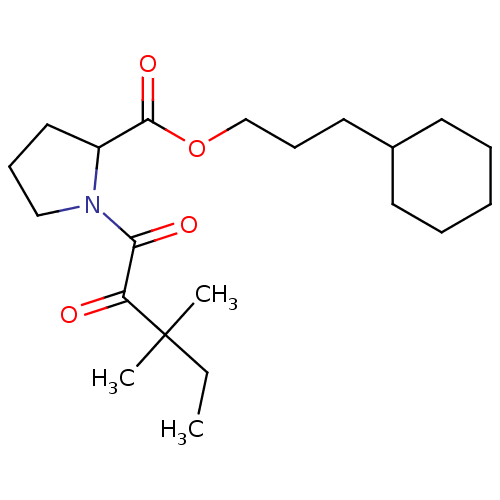
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC1C(=O)OCCCC1CCCCC1 Show InChI InChI=1S/C21H35NO4/c1-4-21(2,3)18(23)19(24)22-14-8-13-17(22)20(25)26-15-9-12-16-10-6-5-7-11-16/h16-17H,4-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068604
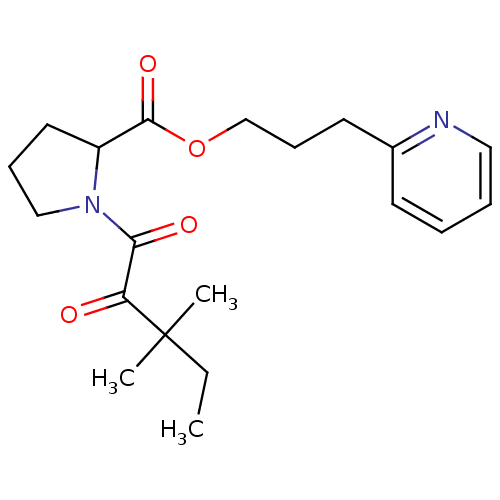
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC1C(=O)OCCCc1ccccn1 Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-13-7-11-16(22)19(25)26-14-8-10-15-9-5-6-12-21-15/h5-6,9,12,16H,4,7-8,10-11,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068600
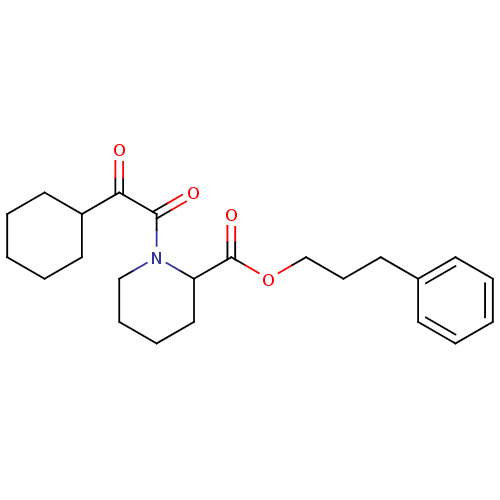
(1-(2-Cyclohexyl-2-oxo-acetyl)-piperidine-2-carboxy...)Show SMILES O=C(OCCCc1ccccc1)C1CCCCN1C(=O)C(=O)C1CCCCC1 Show InChI InChI=1S/C23H31NO4/c25-21(19-13-5-2-6-14-19)22(26)24-16-8-7-15-20(24)23(27)28-17-9-12-18-10-3-1-4-11-18/h1,3-4,10-11,19-20H,2,5-9,12-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068555

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-carb...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1ccccc1 Show InChI InChI=1S/C22H31NO4/c1-4-22(2,3)19(24)20(25)23-15-9-8-14-18(23)21(26)27-16-10-13-17-11-6-5-7-12-17/h5-7,11-12,18H,4,8-10,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data