

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50062005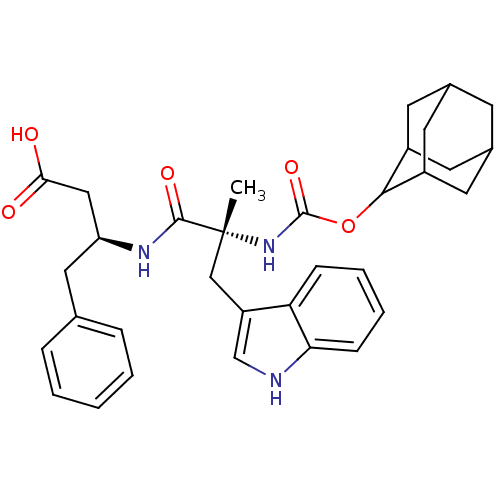 ((S)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM81962 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092154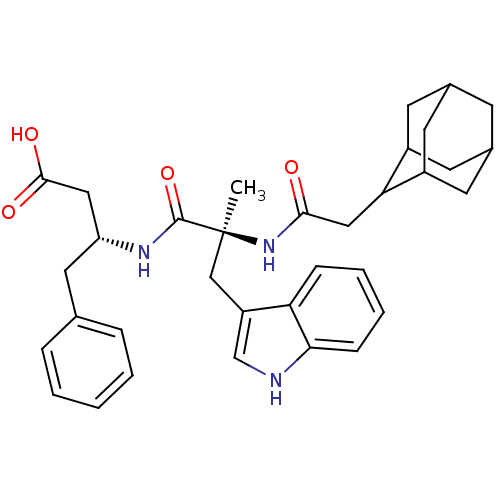 (3-[2-(2-Adamantan-2-yl-acetylamino)-3-(1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061220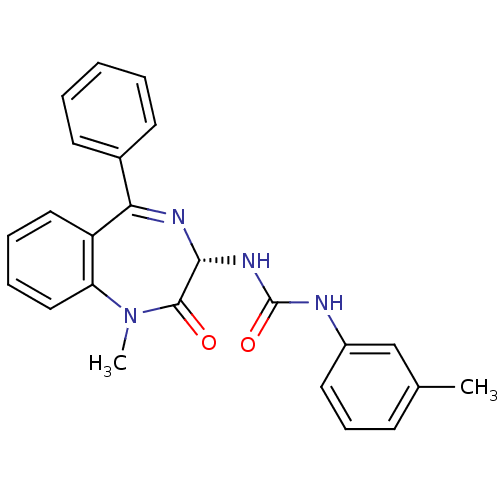 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060321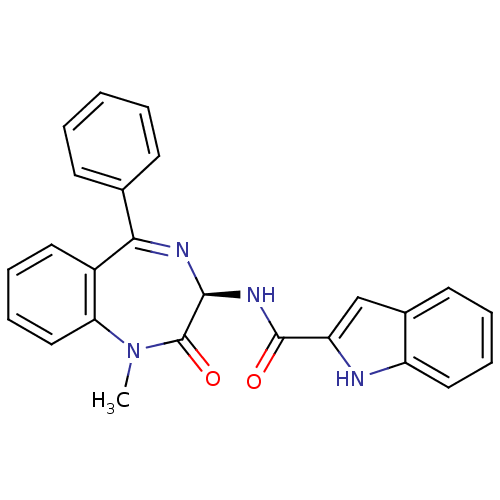 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50092155 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50062005 ((S)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50061220 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50092154 (3-[2-(2-Adamantan-2-yl-acetylamino)-3-(1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM81962 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50092155 ((4S,5R)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||