

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098868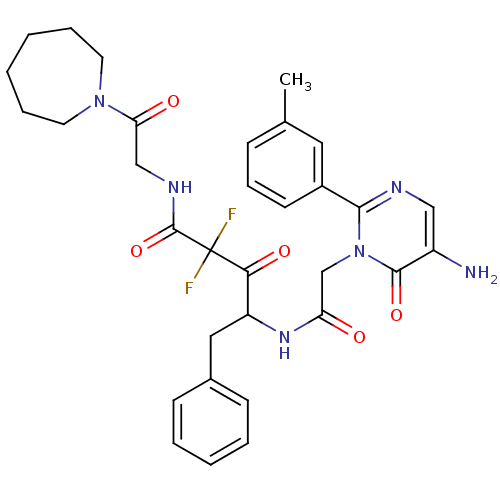 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068894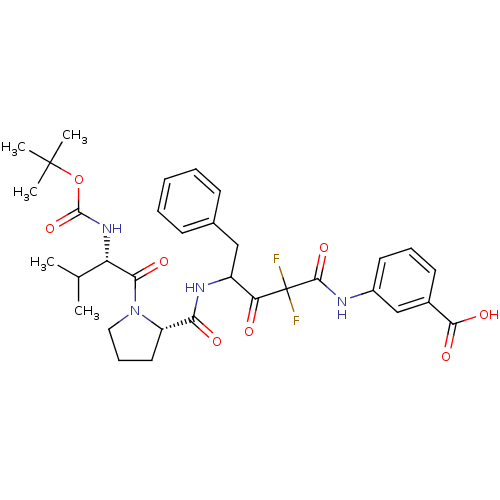 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098892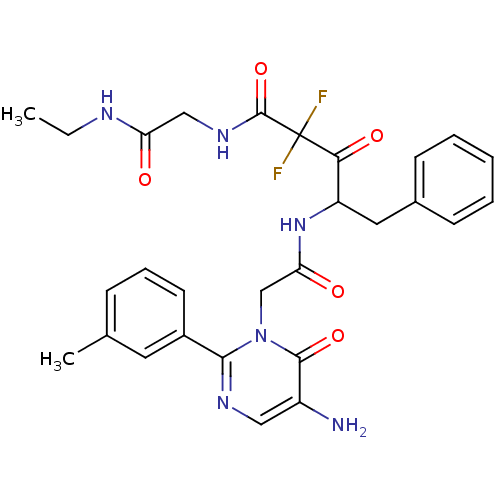 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098879 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM87059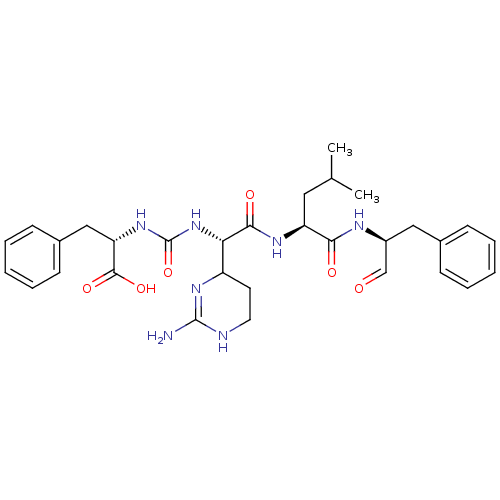 (CHEMBL247767 | Chymostatin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098880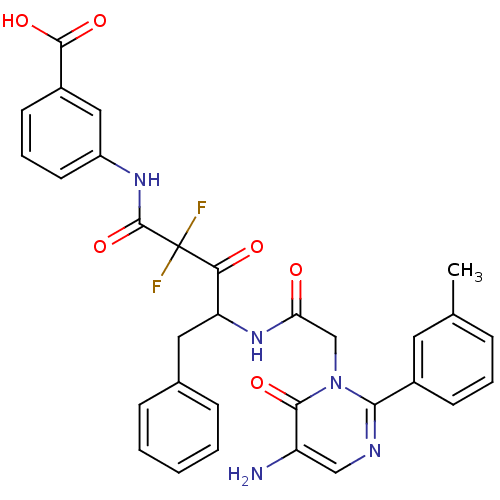 (3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098878 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098870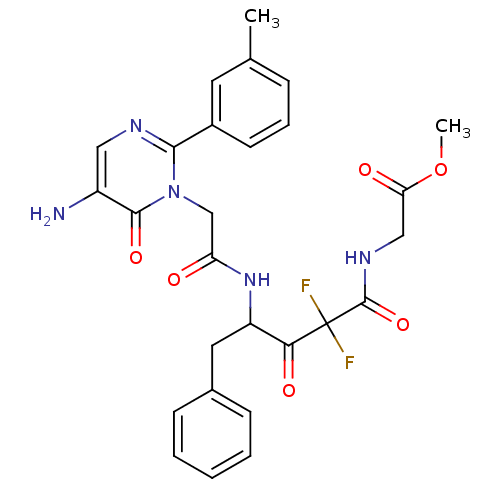 (CHEMBL26182 | {4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098875 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098882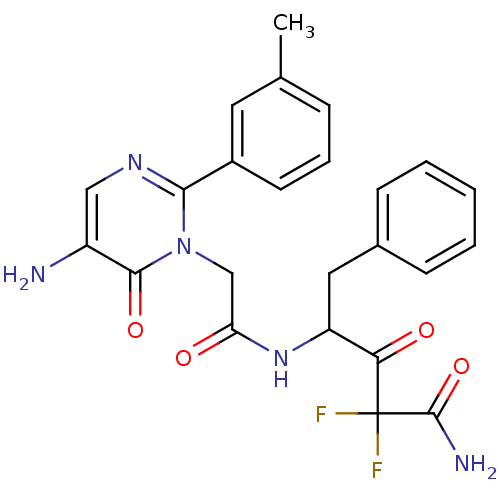 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098882 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098877 (4-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098876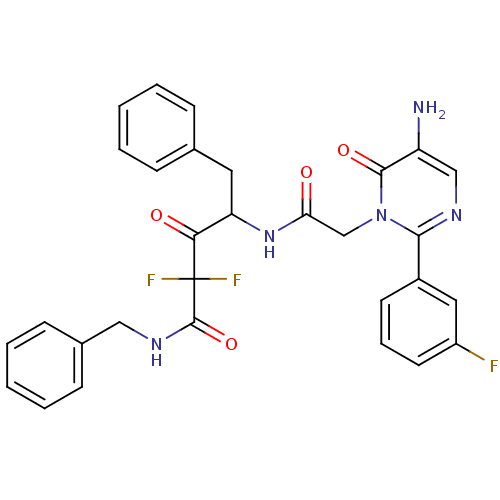 (4-{2-[5-Amino-2-(3-fluoro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098866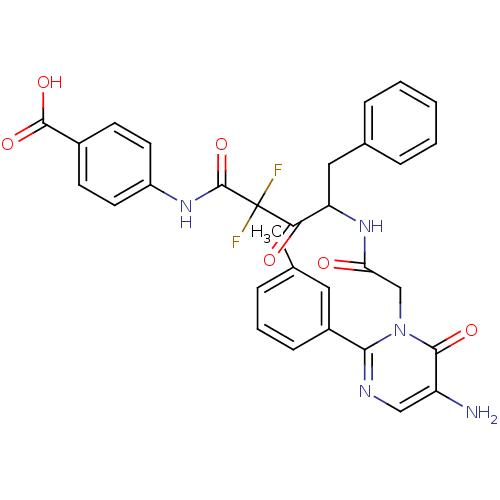 (4-({4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098872 (CHEMBL442146 | {4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098891 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098889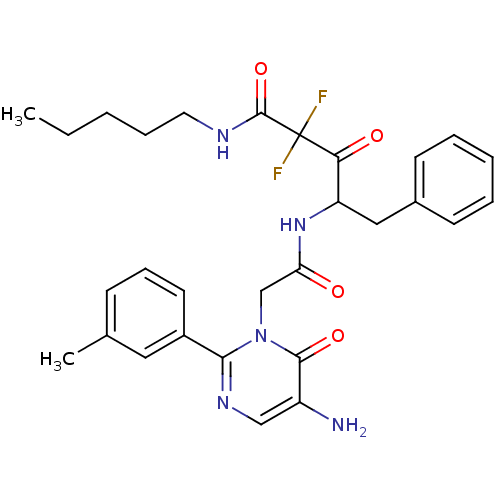 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098867 (3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against canine skin chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098881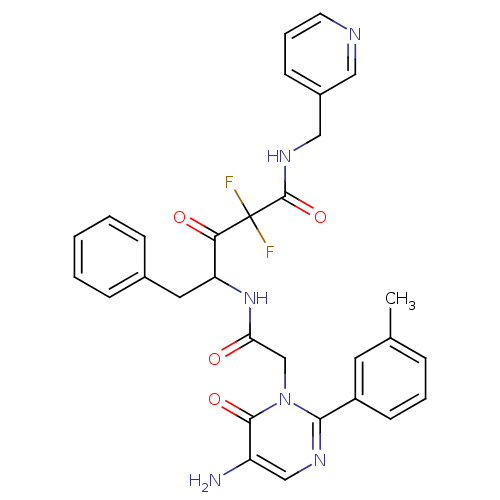 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098886 (4-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098883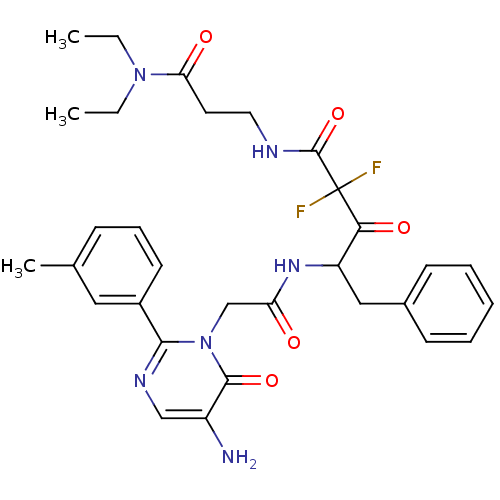 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098888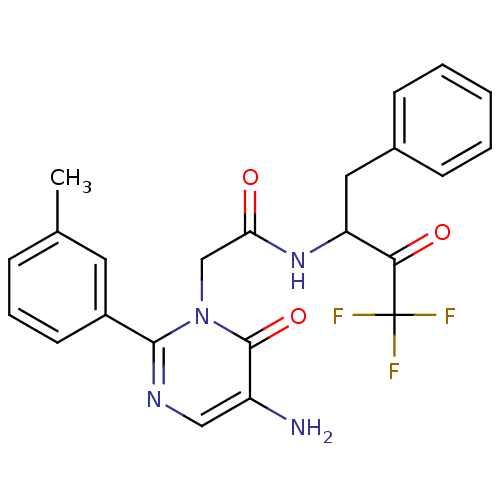 (2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-N-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098884 (4-[2-(5-Amino-6-oxo-2-pyridin-3-yl-6H-pyrimidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 74.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098885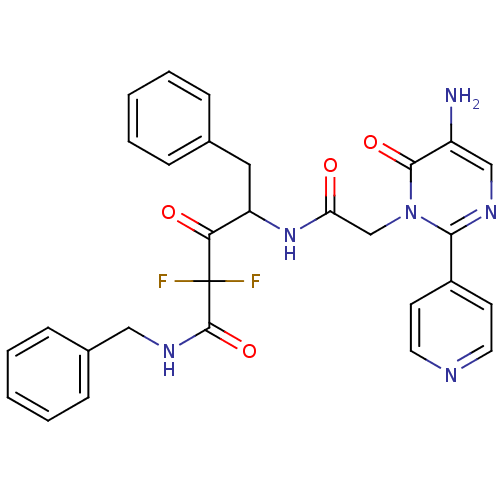 (4-[2-(5-Amino-6-oxo-2-pyridin-4-yl-6H-pyrimidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098871 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098877 (4-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyrimi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098870 (CHEMBL26182 | {4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098884 (4-[2-(5-Amino-6-oxo-2-pyridin-3-yl-6H-pyrimidin-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098886 (4-[2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-ac...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098885 (4-[2-(5-Amino-6-oxo-2-pyridin-4-yl-6H-pyrimidin-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098866 (4-({4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098883 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098876 (4-{2-[5-Amino-2-(3-fluoro-phenyl)-6-oxo-6H-pyrimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098871 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50068894 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 364 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098890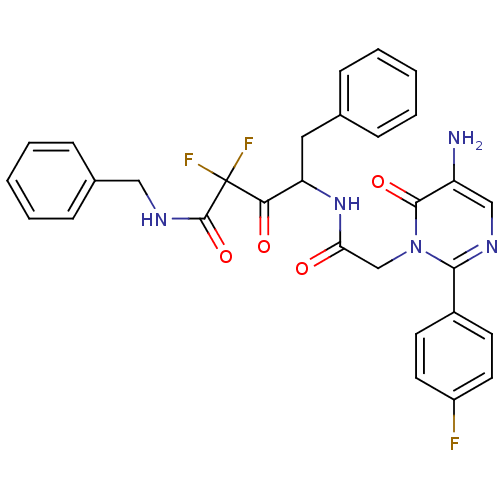 (4-{2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098889 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098888 (2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-N-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098869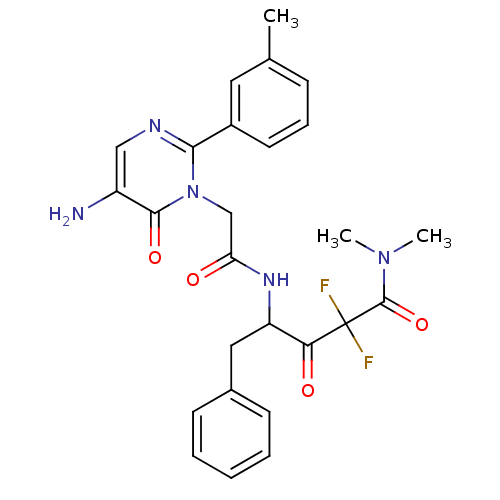 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 489 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098880 (3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098881 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell protease 9 (Mus musculus) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against mouse peritoneal chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against human cathepsin G | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098892 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098867 (3-{4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50098875 (4-[2-(5-Amino-6-oxo-2-m-tolyl-6H-pyrimidin-1-yl)-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against bovine pancreas chymotrypsin | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |