

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507212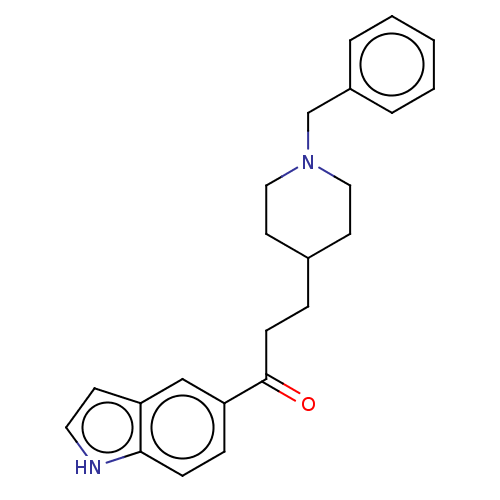 (CHEMBL4585990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507209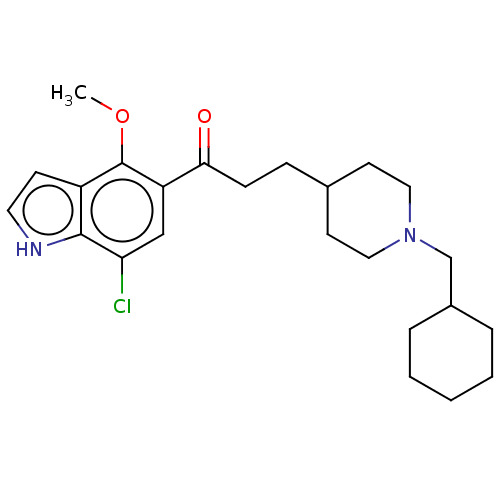 (CHEMBL4537101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507211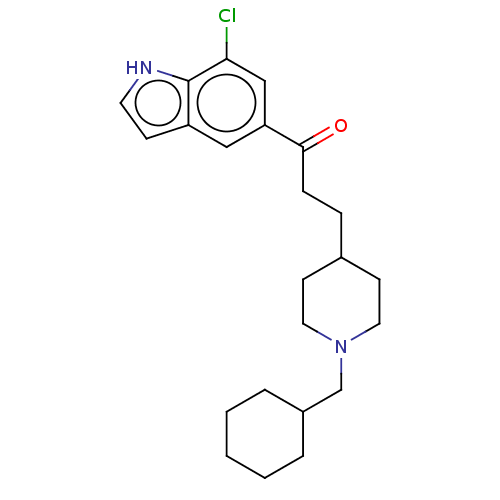 (CHEMBL4444843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507208 (CHEMBL4526050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Agonist activity at 5-HT4R (unknown origin) | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507215 (CHEMBL4562793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507211 (CHEMBL4444843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507213 (CHEMBL4529710) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507209 (CHEMBL4537101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507214 (CHEMBL4567394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507210 (CHEMBL4454706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507208 (CHEMBL4526050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507212 (CHEMBL4585990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507212 (CHEMBL4585990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507208 (CHEMBL4526050) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507211 (CHEMBL4444843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507210 (CHEMBL4454706) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507213 (CHEMBL4529710) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507216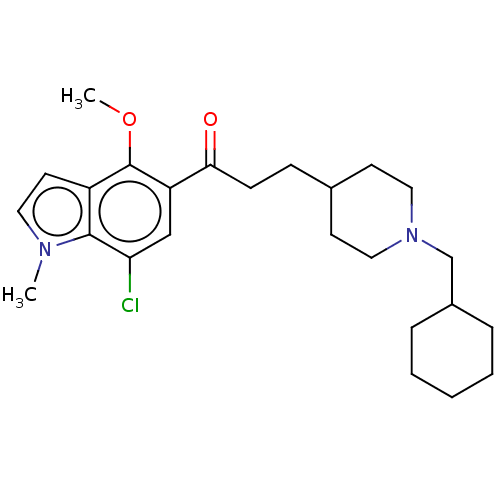 (CHEMBL4559904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507209 (CHEMBL4537101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507215 (CHEMBL4562793) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507220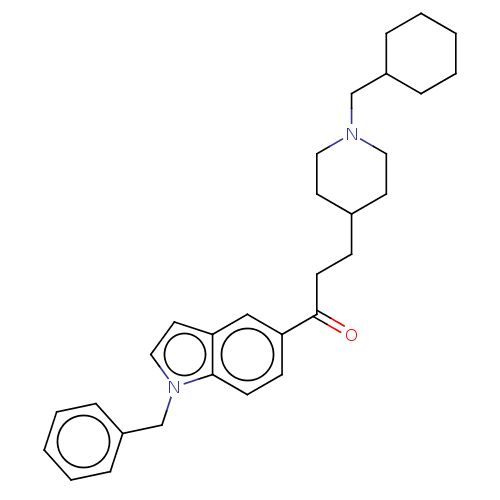 (CHEMBL4578844) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507217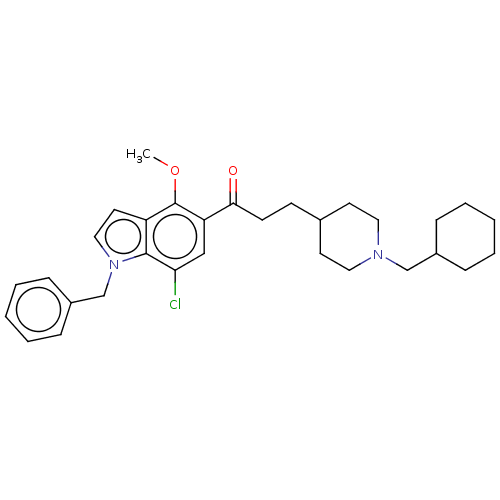 (CHEMBL4541090) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507219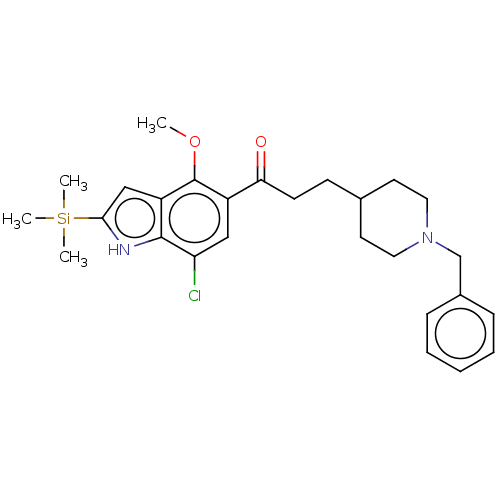 (CHEMBL4540355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507218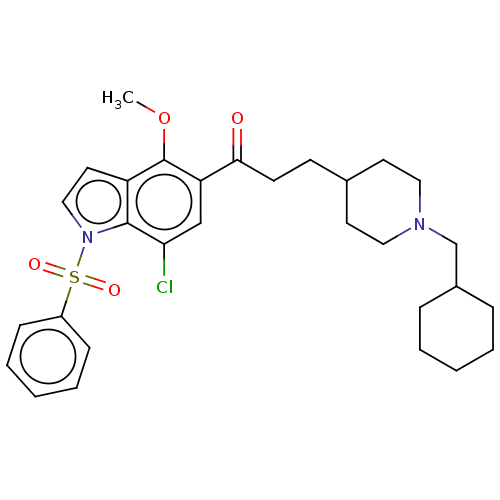 (CHEMBL4583738) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 559 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50507214 (CHEMBL4567394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 716 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at ... | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||