

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526945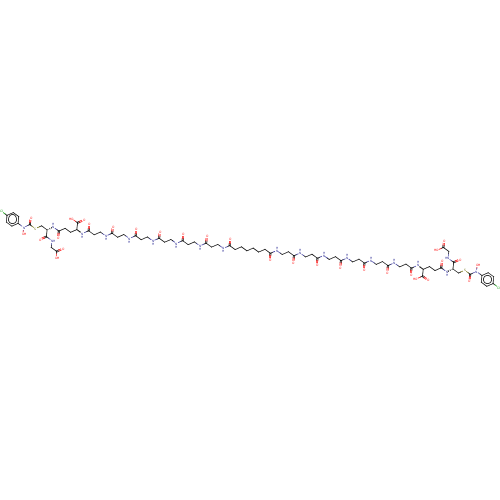 (CHEMBL4473806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526943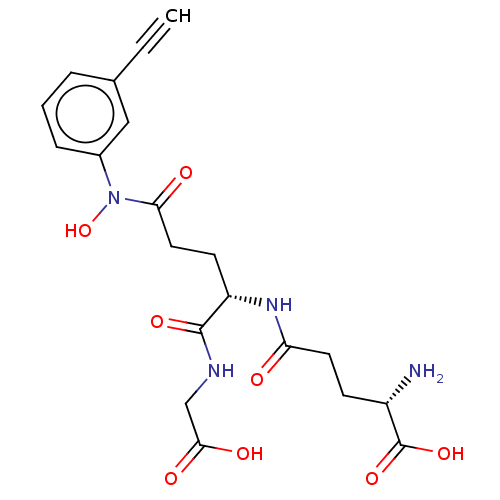 (CHEMBL4436073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526944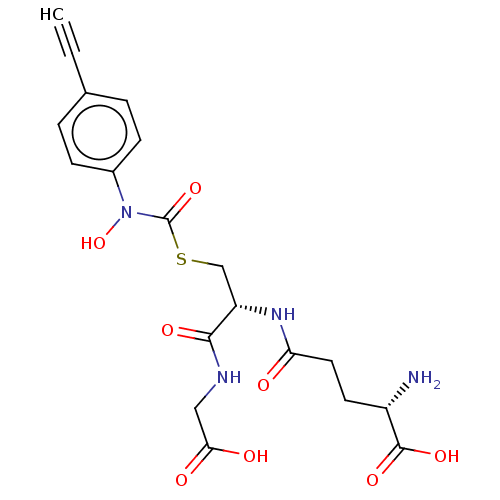 (CHEMBL4450158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526942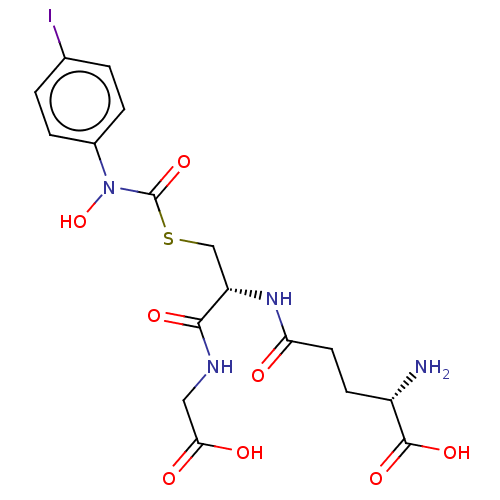 (CHEMBL4438930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826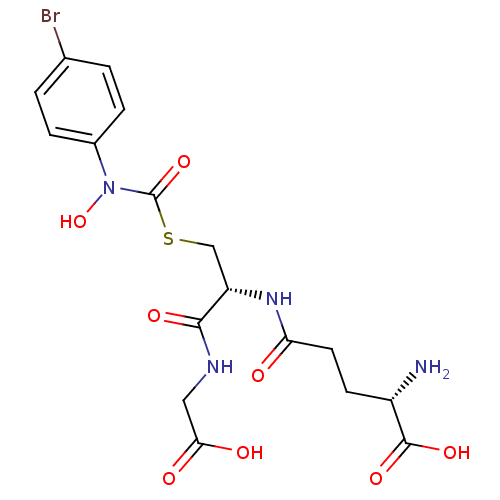 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526948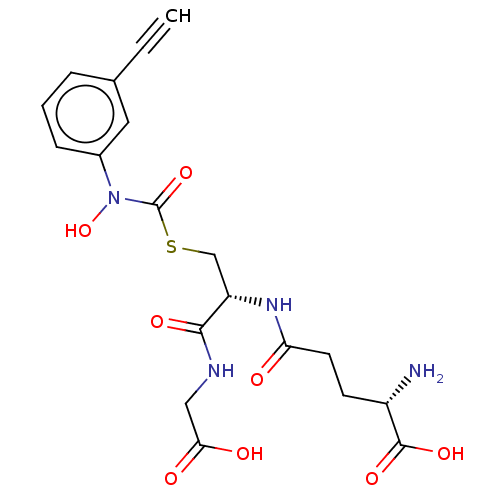 (CHEMBL4559486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824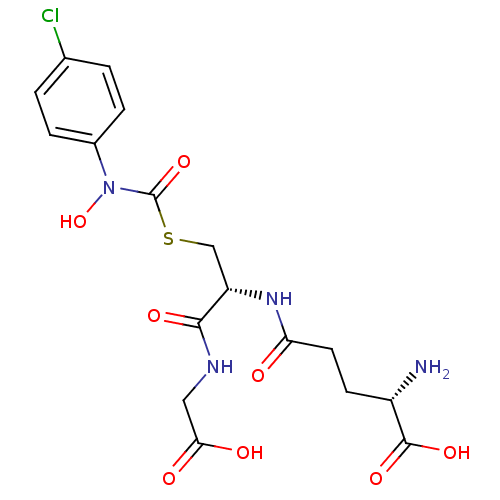 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Mus musculus) | BDBM50526947 (CHEMBL4584432) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse His-tagged Glyoxalase-1 using GSH and MGO as substrate by spectrophotometric method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825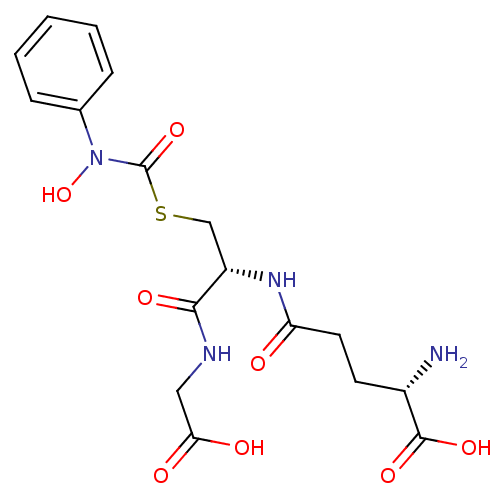 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121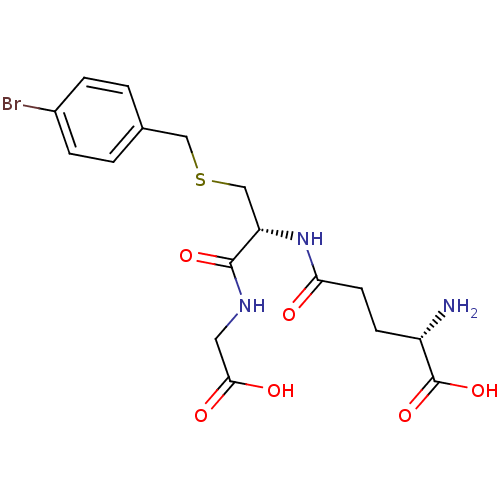 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of Glyoxalase-1 (unknown origin) using GSH and MGO as substrates preincubated with substrates for 6 mins followed by enzyme addition by sp... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Mus musculus) | BDBM50517464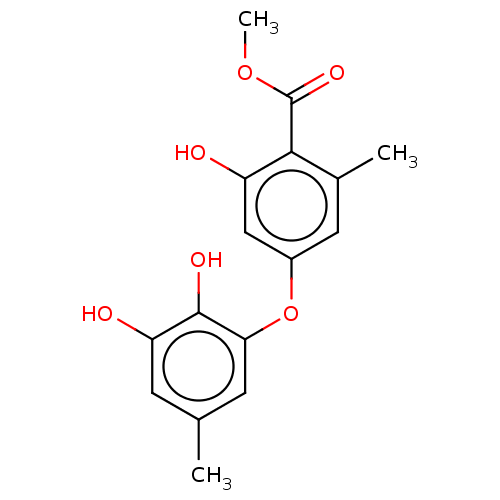 (CHEMBL1234300) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse His-tagged Glyoxalase-1 using GSH and MGO as substrate by spectrophotometric method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Mus musculus) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of recombinant mouse Glyoxalase-1 expressed in Escherichia coli BL21 (DE3) pLysS cells using GSH and MGO as substrate by Dixon plot | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526946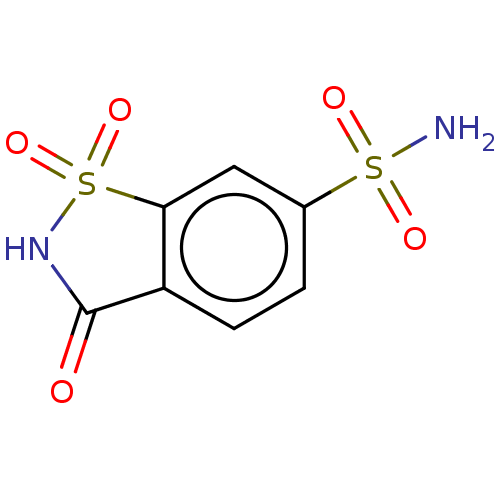 (CHEMBL4456920) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of Glyoxalase-1 (unknown origin) | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||