

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446471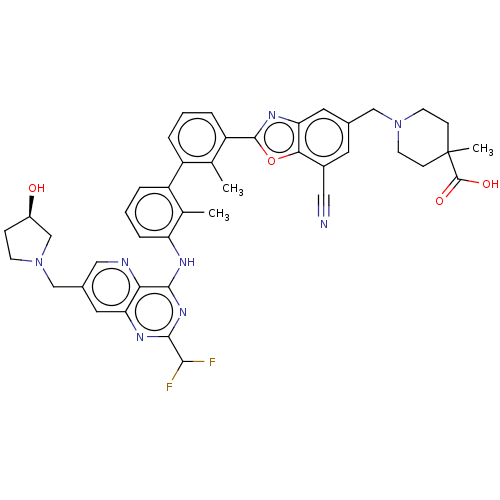 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446465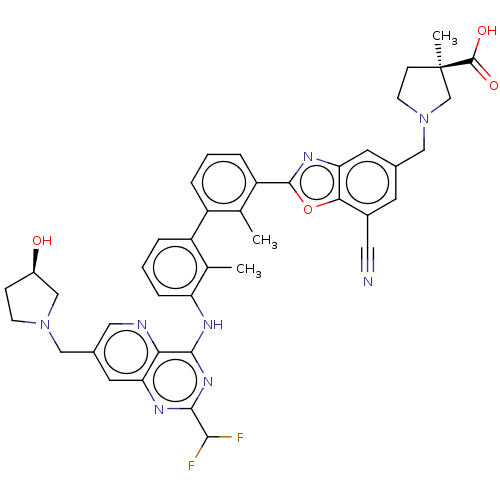 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446473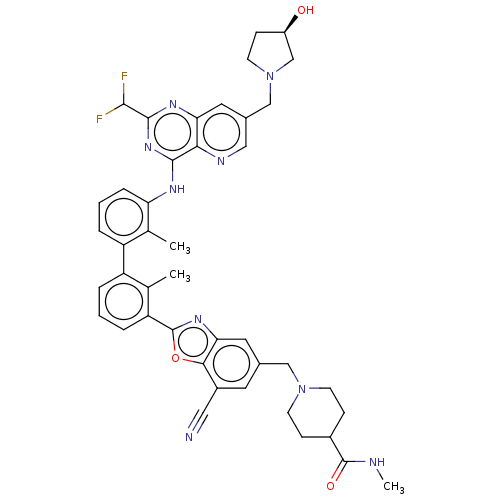 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446474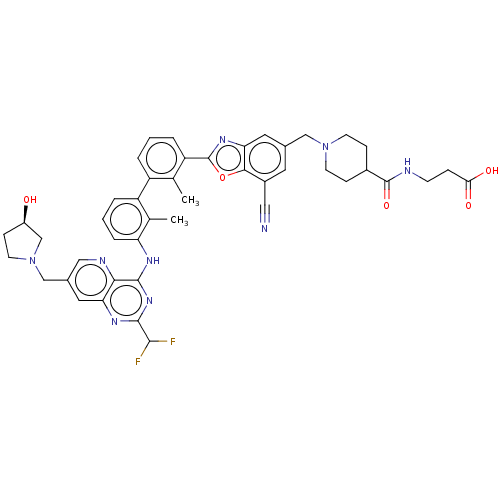 ((R)-3-(1-((7-cyano-2-(3′-(2-(difluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446475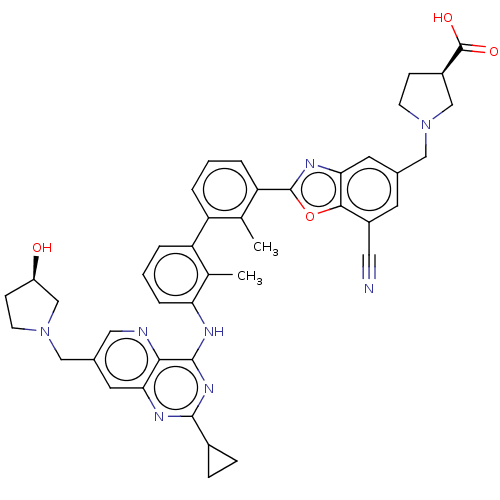 ((R)-1-((7-cyano-2-(3′-(2-cyclopropyl-7-(((R)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446472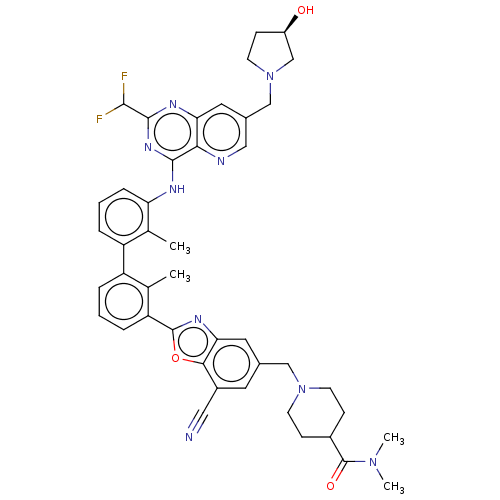 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446462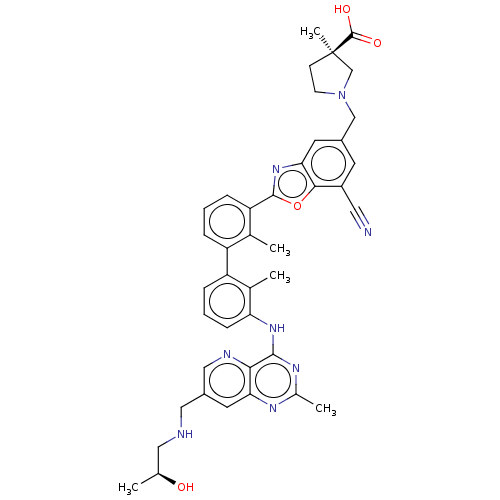 ((R)-1-((7-cyano-2-(3′-(7-(((S)-2-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446476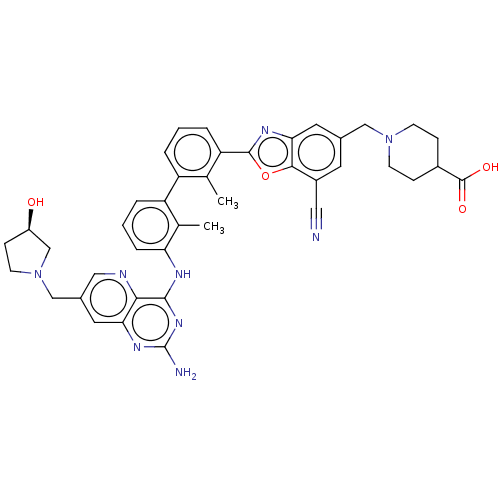 ((R)-1-((2-(3′-(2-amino-7-((3-hydroxypyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446476 ((R)-1-((2-(3′-(2-amino-7-((3-hydroxypyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446477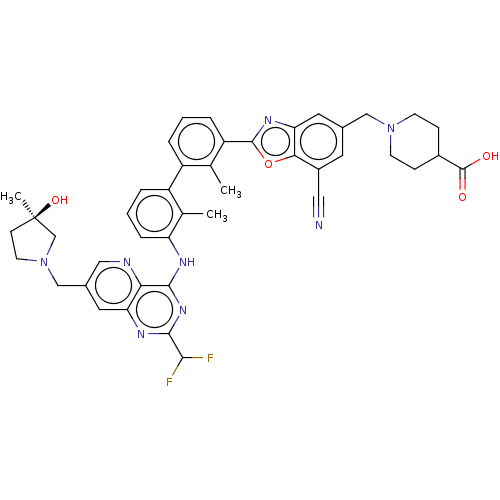 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446477 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446478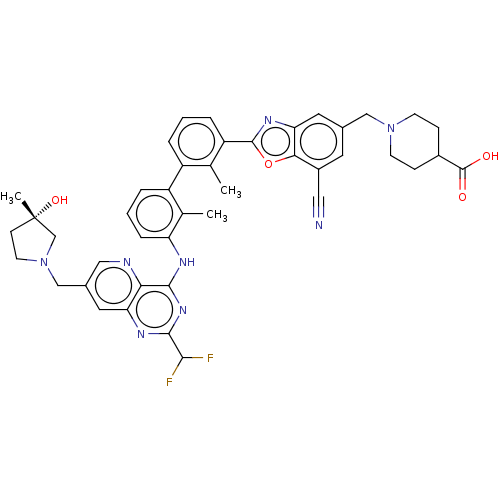 ((S)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446478 ((S)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446479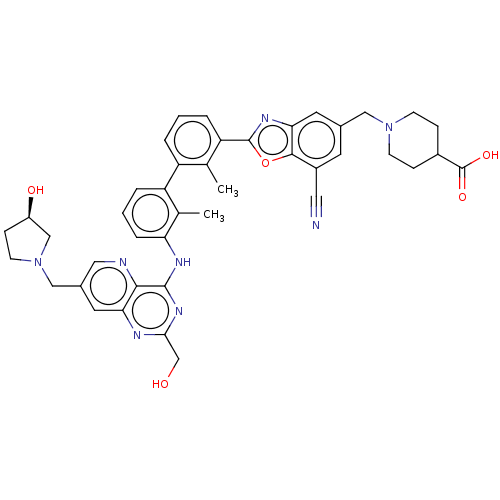 ((R)-1-((7-cyano-2-(3′-(2-(hydroxymethyl)-7-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446453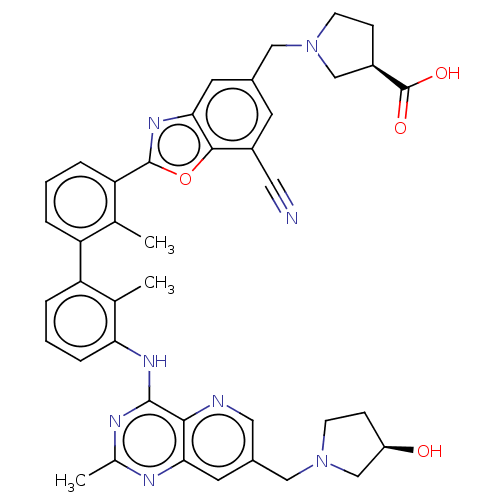 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxypyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446481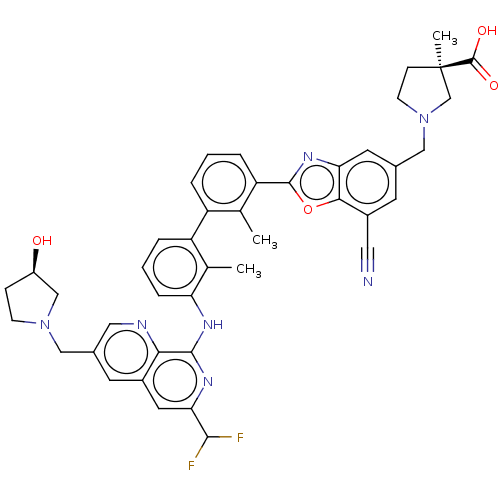 ((R)-1-((7-cyano-2-(3′-(6-(difluoromethyl)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446448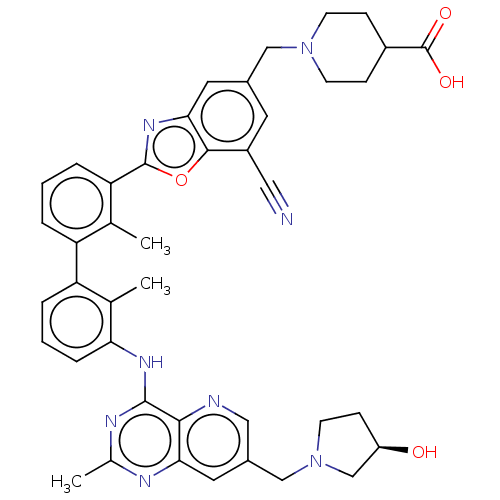 ((R)-1-((7-cyano-2-(3′-(7-((3-hydroxypyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446448 ((R)-1-((7-cyano-2-(3′-(7-((3-hydroxypyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446449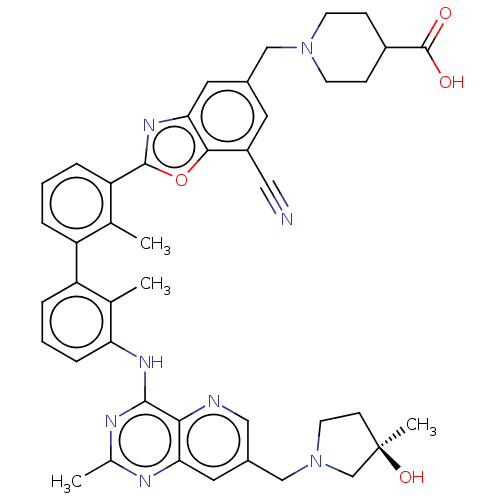 ((R)-1-((7-cyano-2-(3′-(7-((3-hydroxy-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446449 ((R)-1-((7-cyano-2-(3′-(7-((3-hydroxy-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446450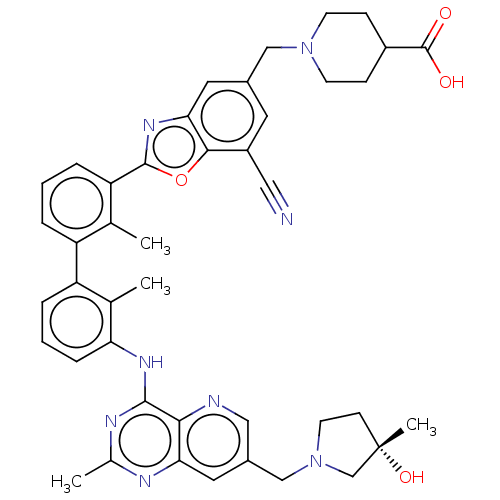 ((S)-1-((7-cyano-2-(3′-(7-((3-hydroxy-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446450 ((S)-1-((7-cyano-2-(3′-(7-((3-hydroxy-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446451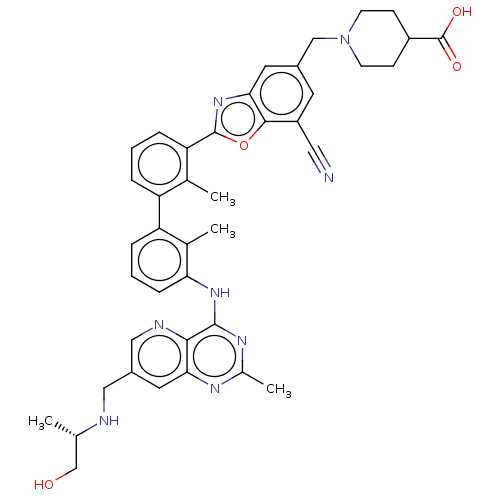 ((S)-1-((7-cyano-2-(3′-(7-((1-hydroxypropan-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446451 ((S)-1-((7-cyano-2-(3′-(7-((1-hydroxypropan-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446452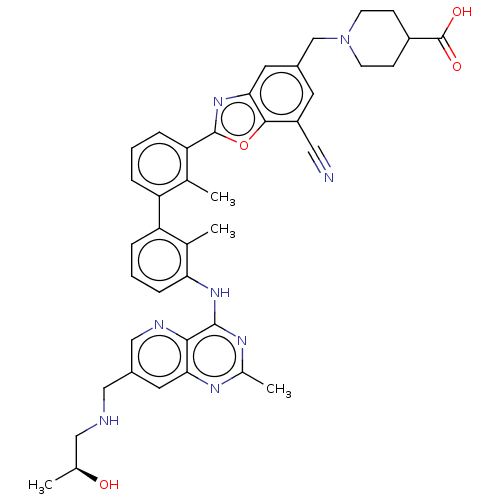 ((S)-1-((7-cyano-2-(3′-(7-((2-hydroxypropylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446453 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxypyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446453 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxypyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446454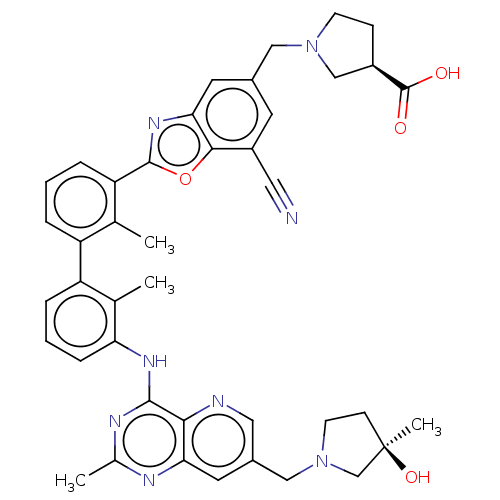 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446454 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446455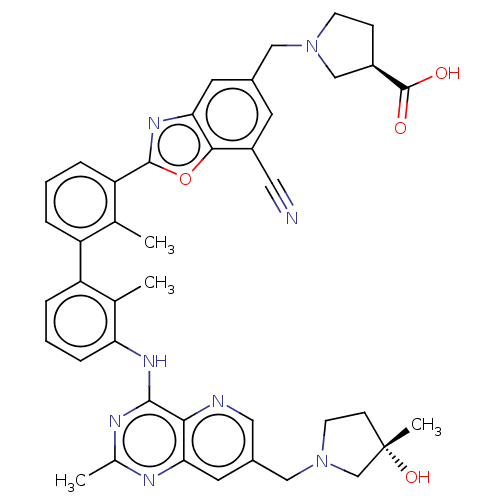 ((R)-1-((7-cyano-2-(3′-(7-(((S)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446455 ((R)-1-((7-cyano-2-(3′-(7-(((S)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446456 ((R)-1-((7-cyano-2-(3′-(7-(((S)-1-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446456 ((R)-1-((7-cyano-2-(3′-(7-(((S)-1-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446457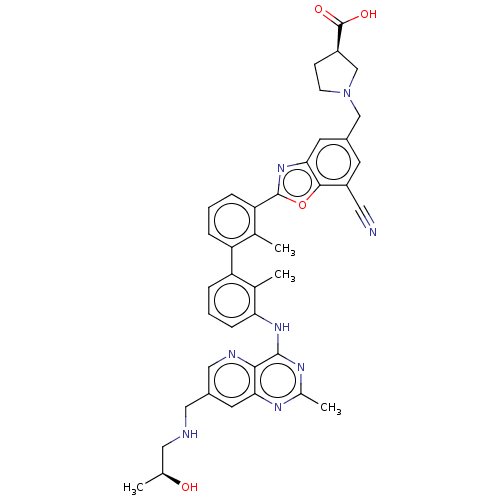 ((R)-1-((7-cyano-2-(3′-(7-(((S)-2-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446458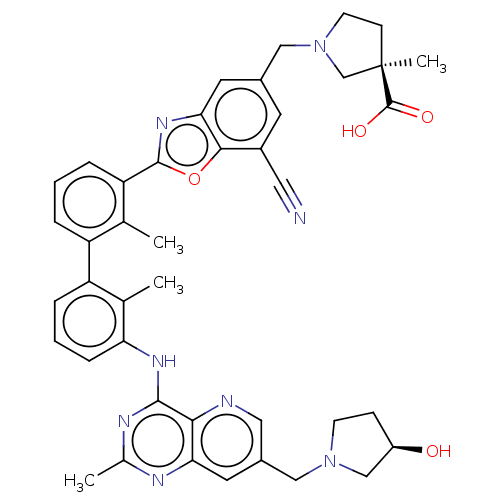 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxypyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446458 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxypyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446459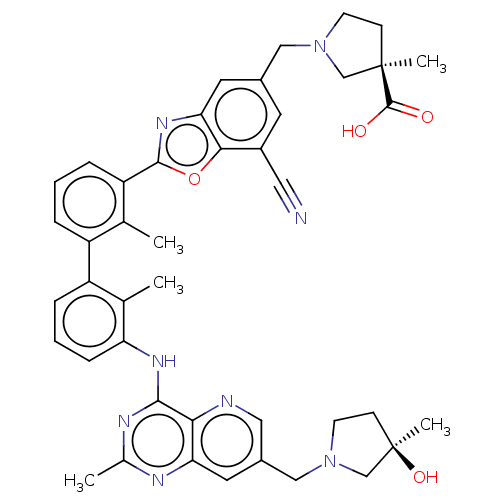 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446459 ((R)-1-((7-cyano-2-(3′-(7-(((R)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446460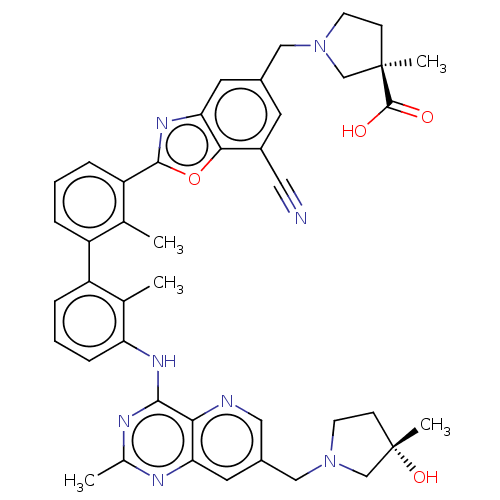 ((R)-1-((7-cyano-2-(3′-(7-(((S)-3-hydroxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446461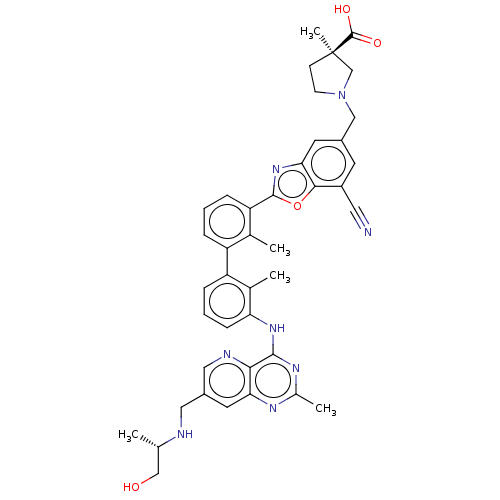 ((R)-1-((7-cyano-2-(3′-(7-(((S)-1-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446461 ((R)-1-((7-cyano-2-(3′-(7-(((S)-1-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446462 ((R)-1-((7-cyano-2-(3′-(7-(((S)-2-hydroxyprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446463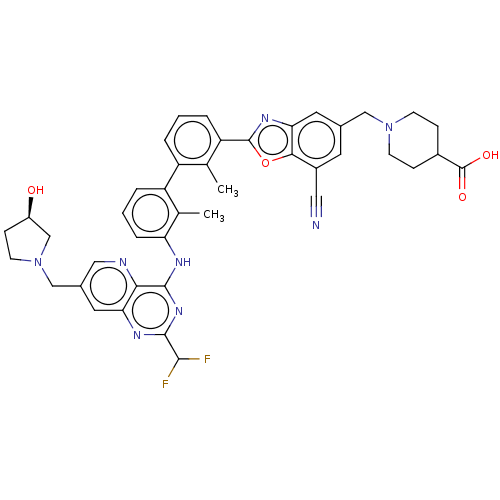 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446463 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446464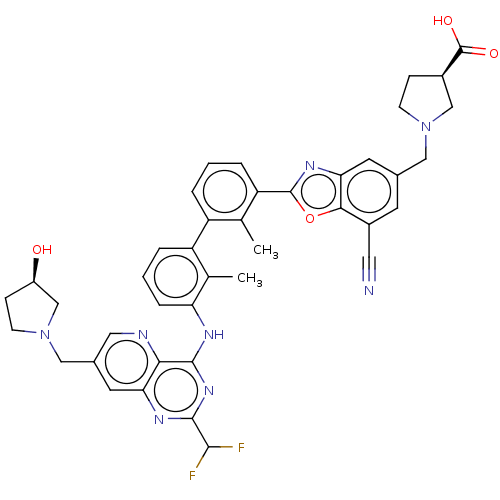 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446464 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446465 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446463 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM446463 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description U2OS/PD-L1 cells (DiscoveRx Corporation) were maintained in McCoy's 5A medium with addition of 10% FBS, 0.25 μg/ml Puromycin. After removing... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM446464 ((R)-1-((7-cyano-2-(3′-(2-(difluoromethyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation US Patent | Assay Description The assays were conducted in a standard black 384-well polystyrene plate with a final volume of 20 μL. Inhibitors were first serially diluted in... | US Patent US10669271 (2020) BindingDB Entry DOI: 10.7270/Q2MG7SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |