

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM36483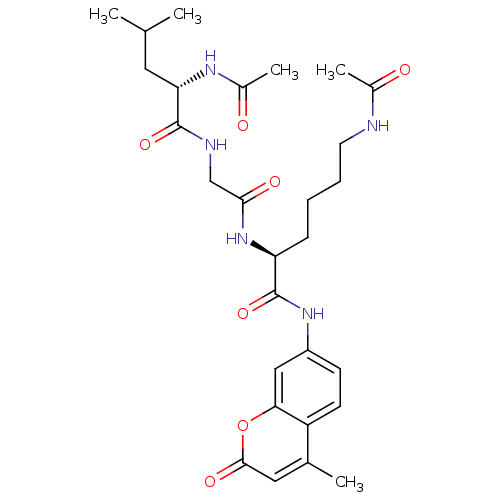 ((S)-6-acetamido-2-(2-((S)-2-acetamido-4-methylpent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | 7.4 | 0 |
Dana-Farber Cancer Institute | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Nat Chem Biol 6: 238-243 (2010) Article DOI: 10.1038/nchembio.313 BindingDB Entry DOI: 10.7270/Q2C53J66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM36484 ((S)-Benzyl (6-acetamido-1-((4-methyl-2-oxo-2H-chro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 643 | n/a | n/a | n/a | n/a | 7.4 | 0 |
Dana-Farber Cancer Institute | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Nat Chem Biol 6: 238-243 (2010) Article DOI: 10.1038/nchembio.313 BindingDB Entry DOI: 10.7270/Q2C53J66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 373 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM178095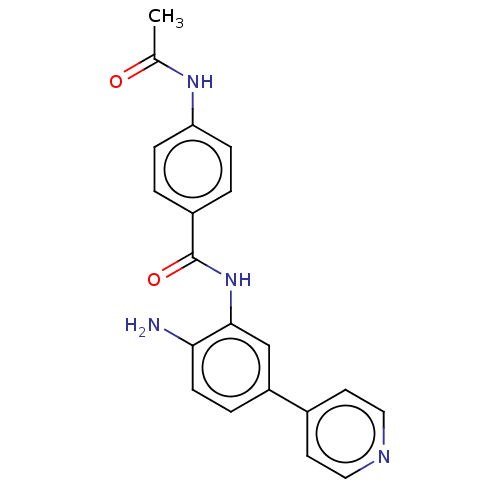 (BRD2492) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM178100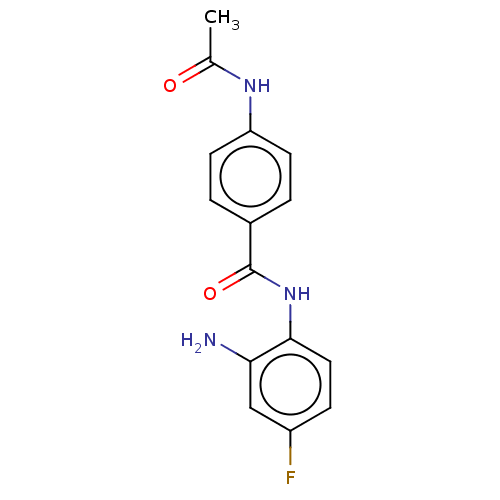 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM49152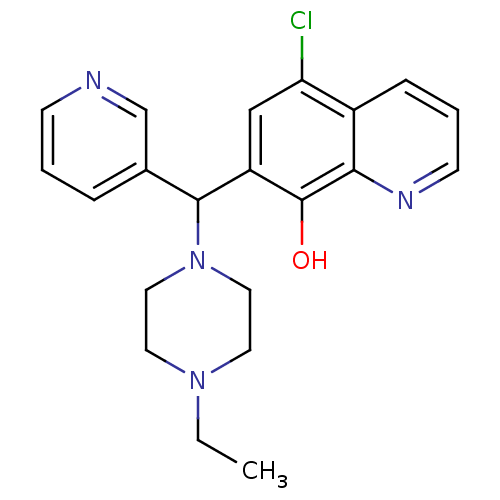 (5-chloranyl-7-[(4-ethylpiperazin-1-yl)-pyridin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Broad Institute | Assay Description Briefly, this fluorogenic assays uses an acetylated lysine tripeptide substrate, amide-linked to a fluorescently quenched aminocoumarin (AMC). Enzyme... | ACS Chem Biol 11: 1844-51 (2016) Article DOI: 10.1021/acschembio.6b00012 BindingDB Entry DOI: 10.7270/Q2QF8RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50276074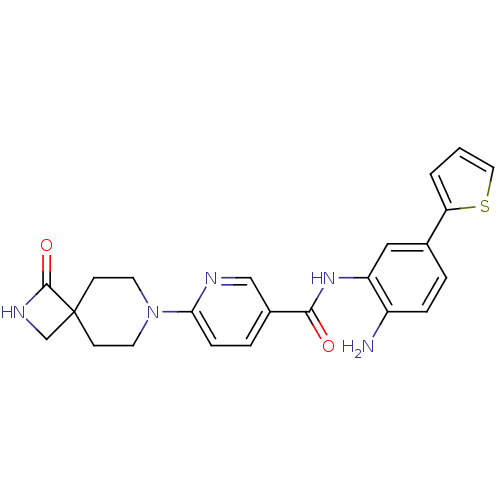 (CHEMBL470791 | HDAC inhibitor, Compound 2 | N-(2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc. | Assay Description Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... | J Biol Chem 288: 26926-43 (2013) Article DOI: 10.1074/jbc.M113.490706 BindingDB Entry DOI: 10.7270/Q2KK99MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19156 ((3S,9S,14aR)-9-benzyl-3-(hept-6-en-1-yl)-6,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19157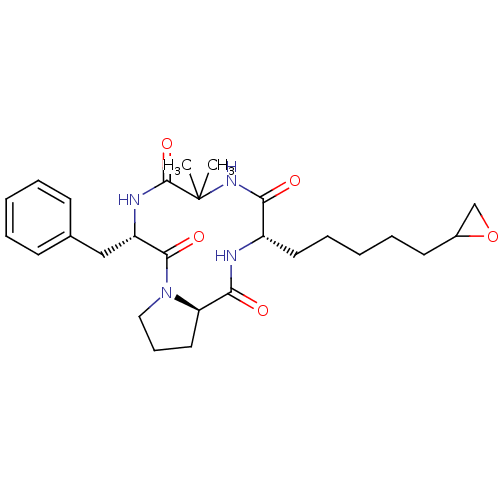 ((3S,9S,14aR)-9-benzyl-6,6-dimethyl-3-[5-(oxiran-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19158 ((3S,9S,14aR)-9-benzyl-3-(7-bromo-6-oxoheptyl)-6,6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59.5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19159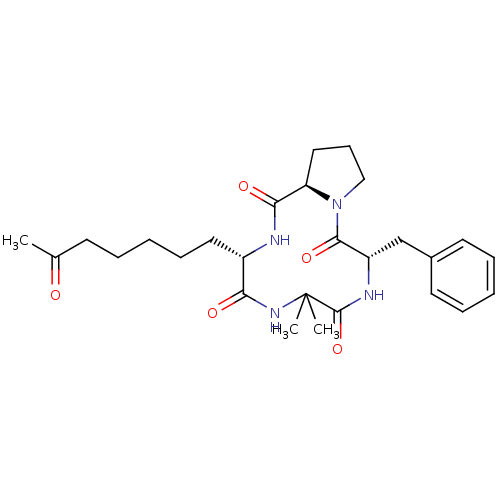 ((3S,9S,14aR)-9-benzyl-6,6-dimethyl-3-(6-oxoheptyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19160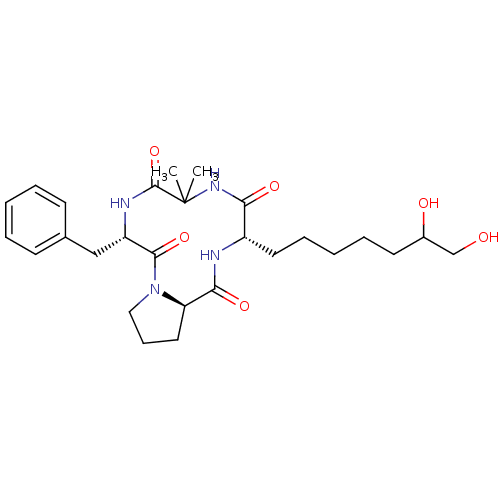 ((3S,9S,14aR)-9-benzyl-3-(6,7-dihydroxyheptyl)-6,6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19161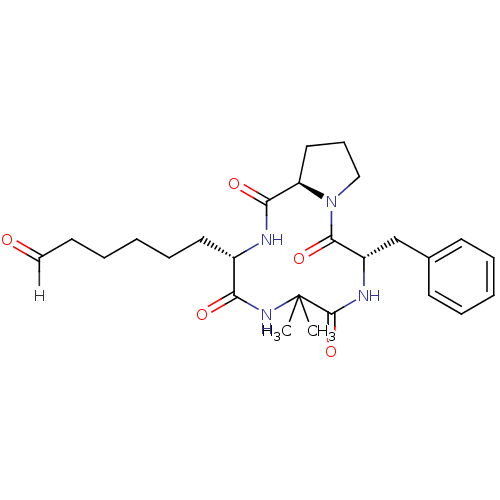 (6-[(3S,9S,14aR)-9-benzyl-6,6-dimethyl-1,4,7,10-tet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19162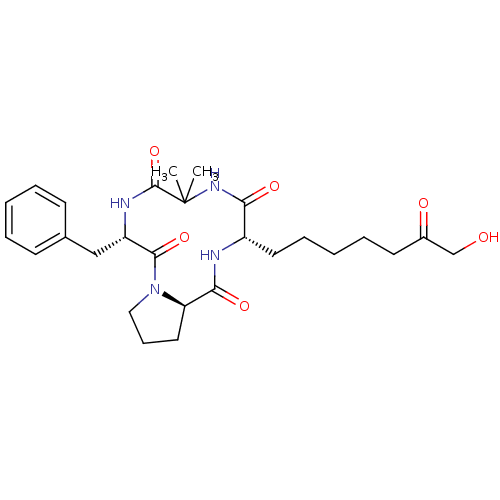 ((3S,9S,14aR)-9-benzyl-3-(7-hydroxy-6-oxoheptyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60.4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Kyushu Institute of Technology | Assay Description For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add... | Bioorg Med Chem 14: 3438-46 (2006) Article DOI: 10.1016/j.bmc.2005.12.063 BindingDB Entry DOI: 10.7270/Q2P55KRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM24624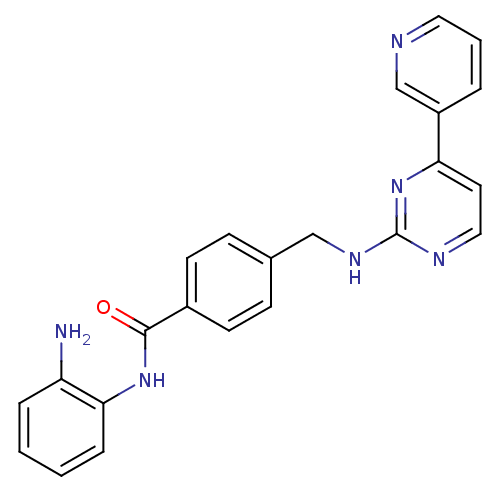 (CHEMBL272980 | MGCD-0103 | MGCD0103 | N-(2-aminoph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
MethylGene Inc. | Assay Description The HDAC enzyme in vitro assay was based on a homogeneous fluorescence release assay. Purified recombinant HDAC enzymes were incubated with compounds... | J Med Chem 51: 4072-5 (2008) Article DOI: 10.1021/jm800251w BindingDB Entry DOI: 10.7270/Q28P5XTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 51: 2350-3 (2008) Article DOI: 10.1021/jm800079s BindingDB Entry DOI: 10.7270/Q2W957H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 51: 2350-3 (2008) Article DOI: 10.1021/jm800079s BindingDB Entry DOI: 10.7270/Q2W957H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM25146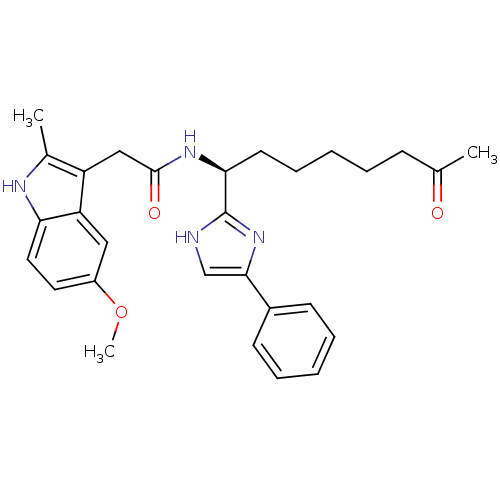 (2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 51: 2350-3 (2008) Article DOI: 10.1021/jm800079s BindingDB Entry DOI: 10.7270/Q2W957H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM25147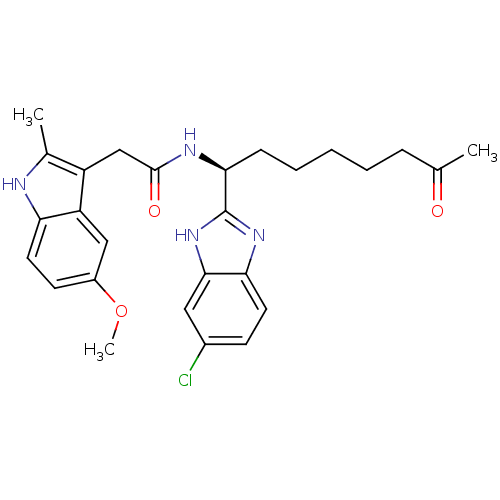 (N-[(1S)-1-(6-chloro-1H-1,3-benzodiazol-2-yl)-7-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 51: 2350-3 (2008) Article DOI: 10.1021/jm800079s BindingDB Entry DOI: 10.7270/Q2W957H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM25148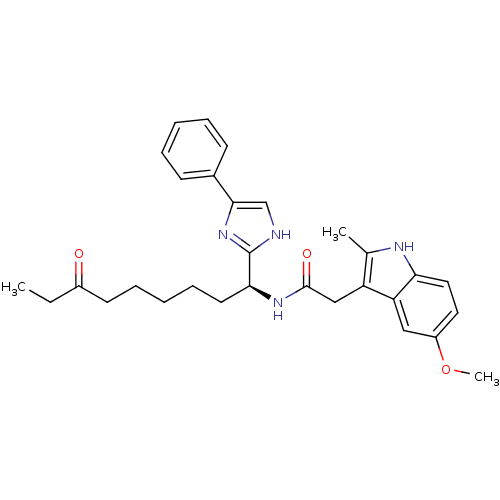 (2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
IRBM/Merck | Assay Description The Fluor-de-Lys HDAC activity assay kit (Biomol) was used. Purified recombinant HDAC enzyme was incubated with Fluor-de-Lys substrate in the presenc... | J Med Chem 51: 2350-3 (2008) Article DOI: 10.1021/jm800079s BindingDB Entry DOI: 10.7270/Q2W957H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50380399 (CHEMBL2018302 | Tubastatin A | US10227295, Compoun...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
The Board of Trustees of The University of Illinois US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US8748451 (2014) BindingDB Entry DOI: 10.7270/Q26D5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM163619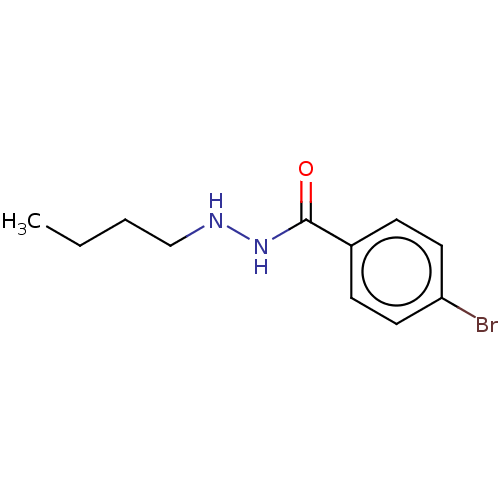 (UF010 | US10807944, Compound UF010 | US11731934, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Florida College of Medicine; Northwest Agriculture and Forestry University | Assay Description Activity against HDACs 1 to 11 was assessed by using an acetylated 7-amino-4-methylcoumarin (AMC)-labeled peptide substrate. A substrate based on res... | Chem Biol 22: 273-84 (2015) Article DOI: 10.1016/j.chembiol.2014.12.015 BindingDB Entry DOI: 10.7270/Q2TX3D48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162728 (US9056843, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162750 (US9056843, 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162779 (US9056843, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162809 (US9056843, 100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162815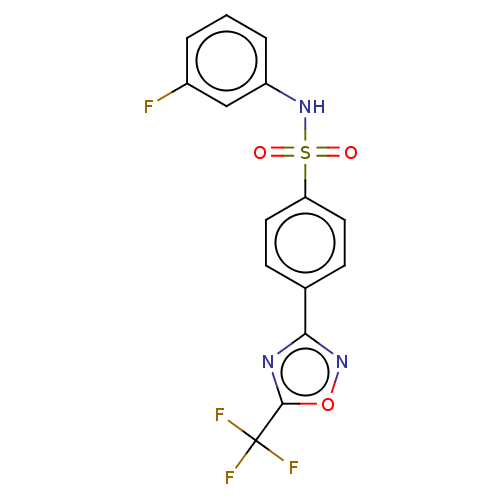 (US9056843, 106) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162842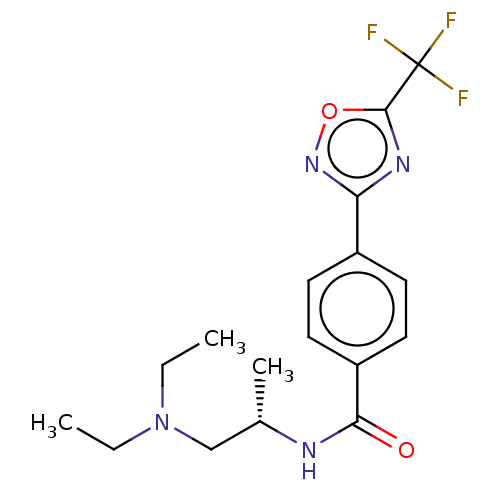 (US9056843, 133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162849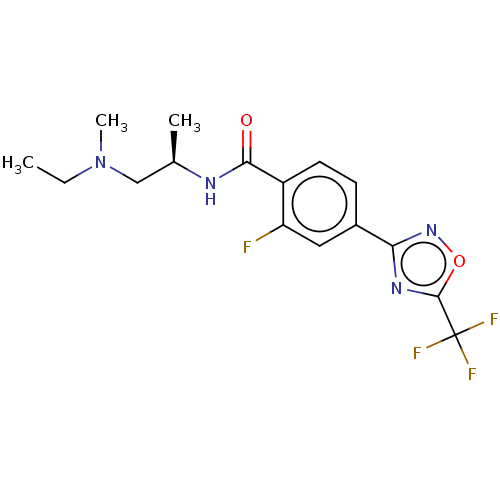 (US9056843, 140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162710 (US9056843, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162711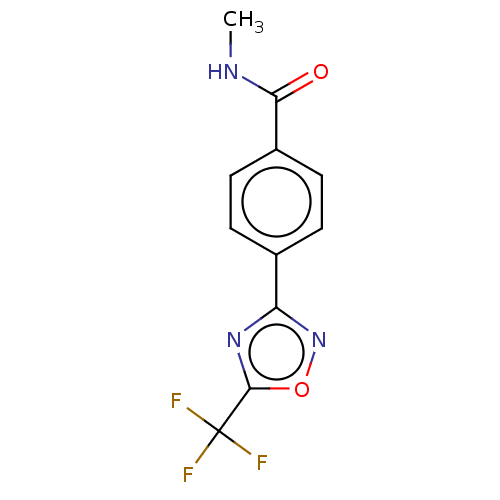 (US9056843, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162712 (US9056843, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162713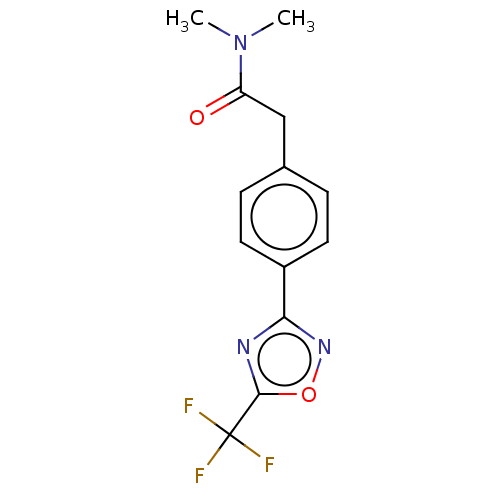 (US9056843, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162714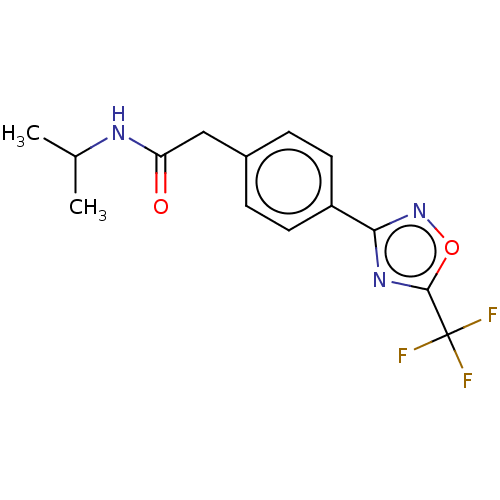 (US9056843, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 970 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162715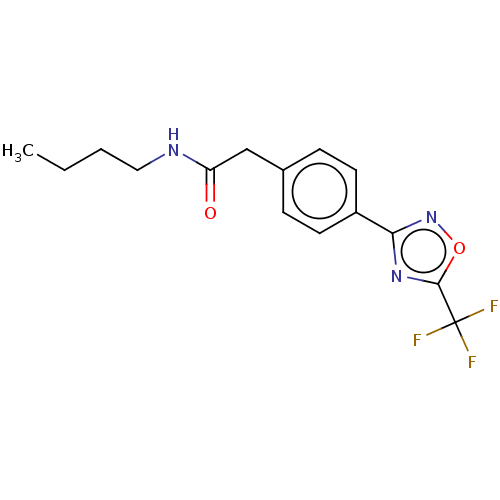 (US9056843, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162716 (US9056843, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 960 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162717 (US9056843, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162718 (US9056843, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162719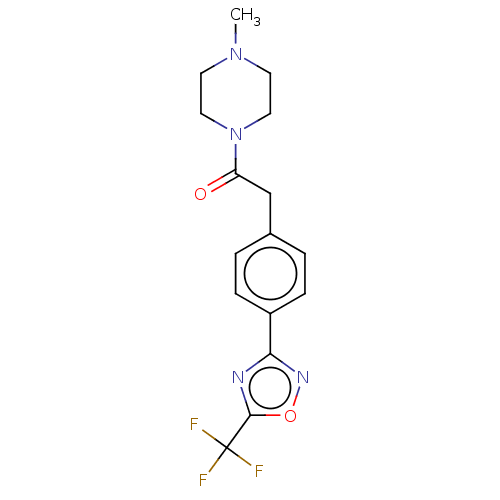 (US9056843, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162720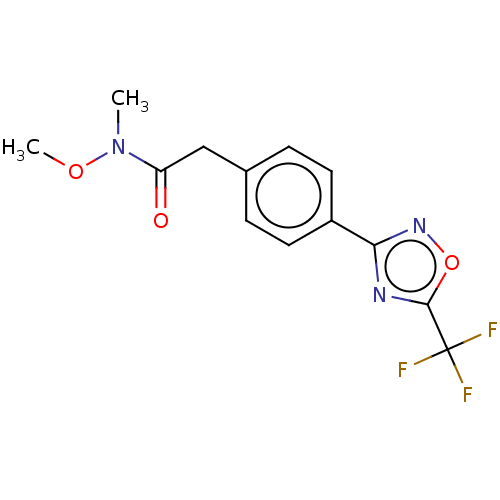 (US9056843, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162721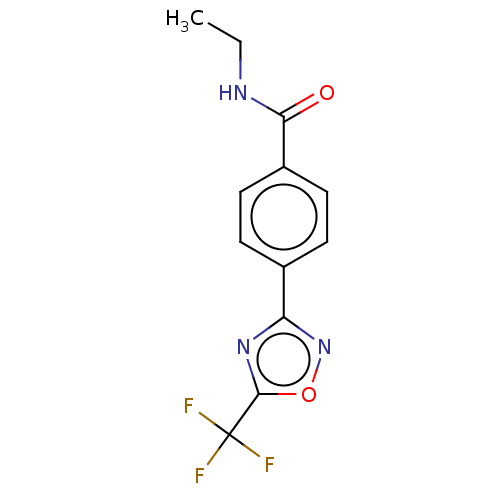 (US9056843, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162722 (US9056843, 13 | US9056843, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM162723 (US9056843, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 8.0 | 25 |
NOVARTIS AG US Patent | Assay Description Human recombinant HDAC4 was expressed in full length form (aa 2-1084) in Sf9 insect cells (obtained from ATCC) using baculovirus generated with Bac-t... | US Patent US9056843 (2015) BindingDB Entry DOI: 10.7270/Q24F1PG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |