

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against serotonin 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc Curated by ChEMBL | Assay Description Effect of the compound on forskolin stimulated adenylate cyclase activity at 5-hydroxytryptamine 1A receptor of guinea pig hippocampus. | J Med Chem 35: 4503-5 (1992) BindingDB Entry DOI: 10.7270/Q29C6WC5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039564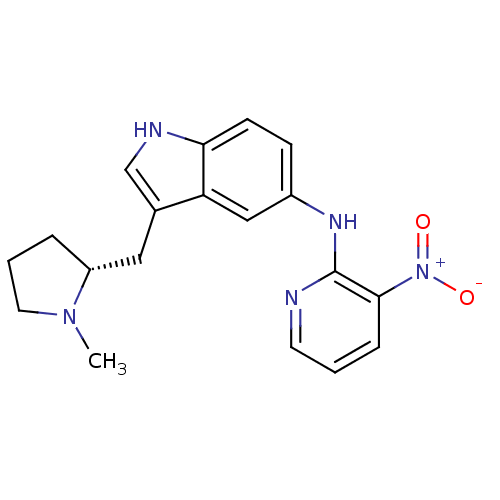 (CHEMBL83597 | [3-((R)-1-Methyl-pyrrolidin-2-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against serotonin 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039566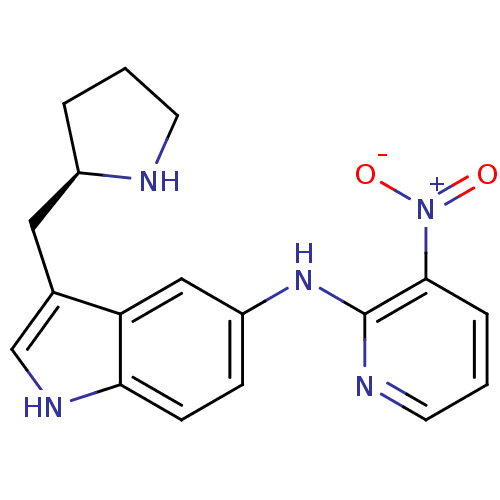 ((3-Nitro-pyridin-2-yl)-(3-(R)-1-pyrrolidin-2-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against serotonin 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039565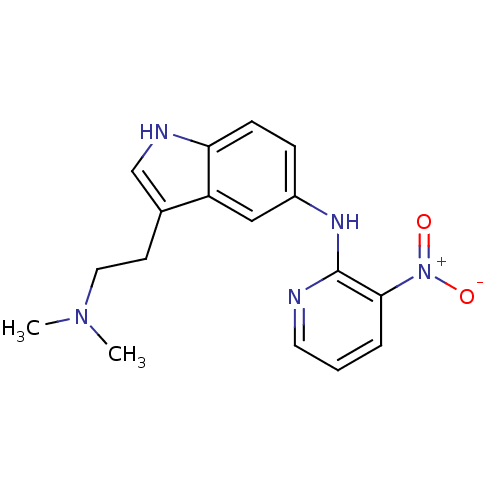 (CHEMBL314213 | [3-(2-Dimethylamino-ethyl)-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against serotonin 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039567 (CHEMBL84942 | [3-(1-Methyl-pyrrolidin-3-yl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against serotonin 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50081704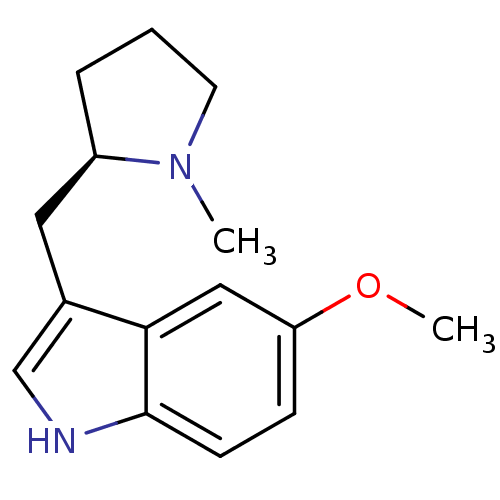 (5-Methoxy-3-((R)-1-methyl-pyrrolidin-2-ylmethyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor | J Med Chem 35: 4503-5 (1992) BindingDB Entry DOI: 10.7270/Q29C6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106707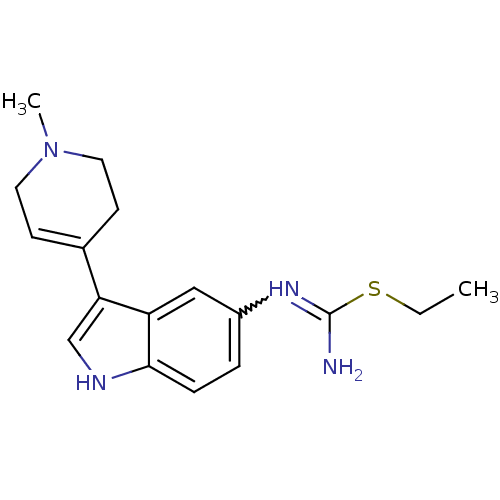 (CHEMBL1957358 | US8586620, 67) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM30707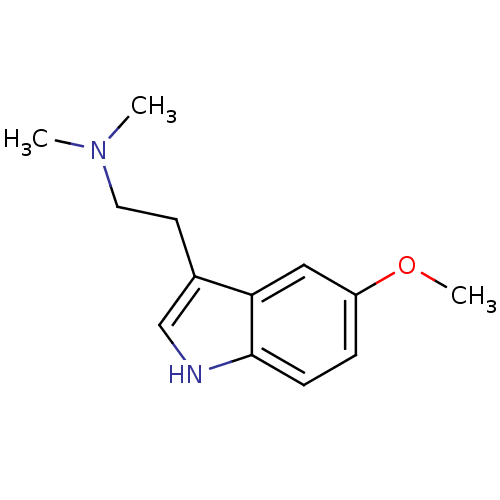 (2-(5-methoxy-1H-indol-3-yl)-N,N-dimethyl-ethanamin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc Curated by ChEMBL | Assay Description Binding affinity of the compound against 5-hydroxytryptamine 1D receptor | J Med Chem 35: 4503-5 (1992) BindingDB Entry DOI: 10.7270/Q29C6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106699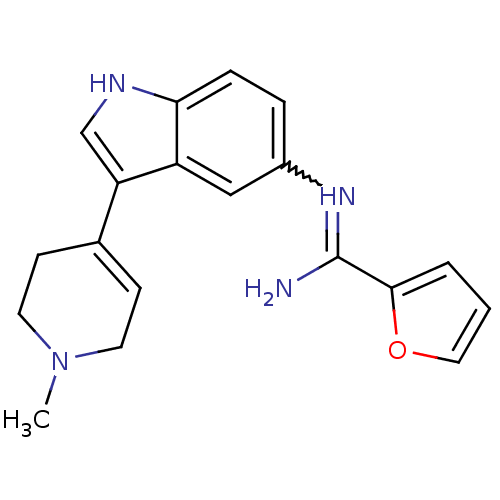 (CHEMBL1957350 | US8586620, 46) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106698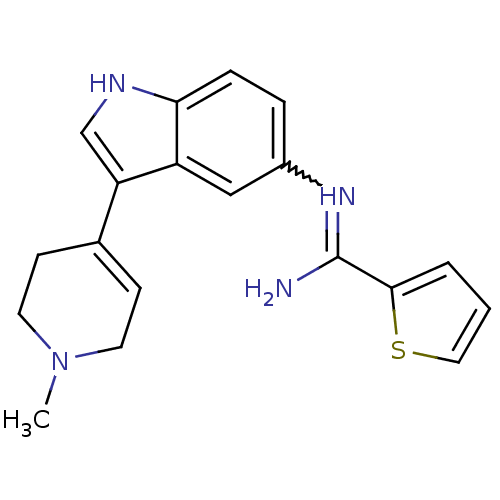 (CHEMBL1957349 | US8586620, 42) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106706 (CHEMBL1957356 | US8586620, 64) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50286672 ((S)-2-(2-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards guinea pig 5-hydroxytryptamine 1D receptor was determined | Bioorg Med Chem Lett 5: 663-666 (1995) Article DOI: 10.1016/0960-894X(95)00091-7 BindingDB Entry DOI: 10.7270/Q2ZK5GMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against serotonin 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086095 (3-{1-[4-Methoxy-3-(4-methyl-piperazin-1-yl)-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106715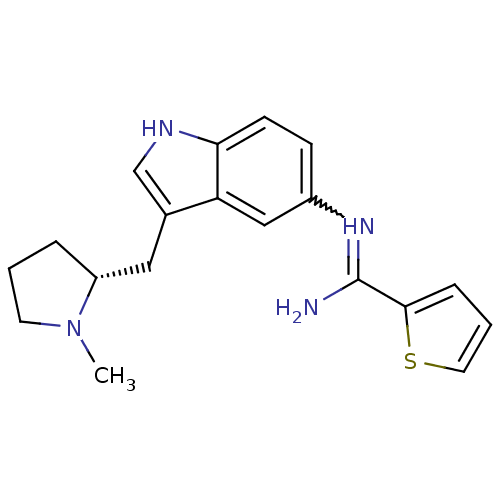 (US8586620, 97) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086097 (1-[2-(5-Fluoro-1H-indol-3-yl)-ethyl]-3-[4-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086104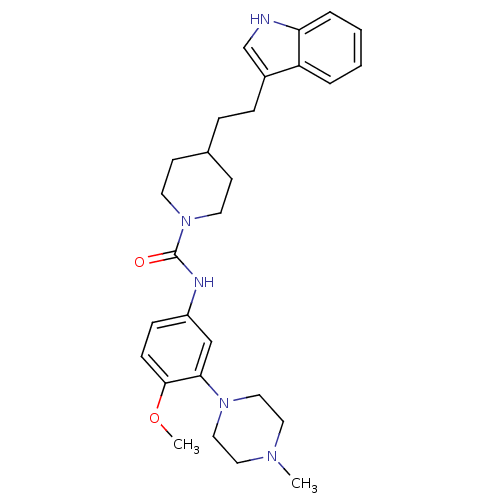 (4-[2-(1H-Indol-3-yl)-ethyl]-piperidine-1-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086102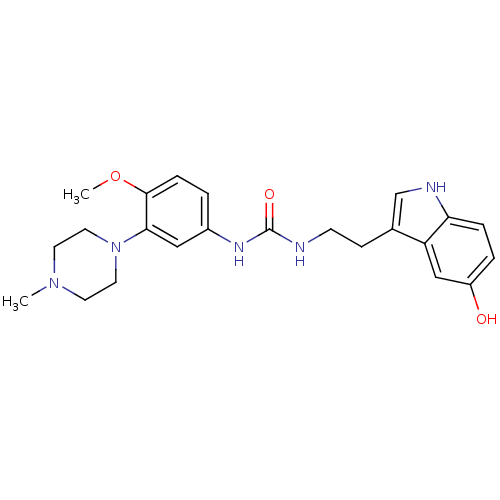 (1-[2-(5-Hydroxy-1H-indol-3-yl)-ethyl]-3-[4-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086112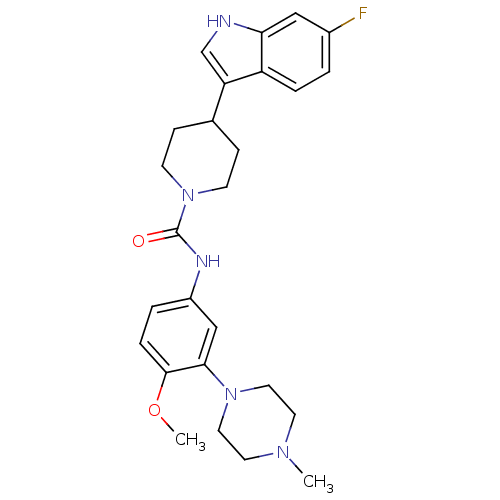 (4-(6-Fluoro-1H-indol-3-yl)-piperidine-1-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086114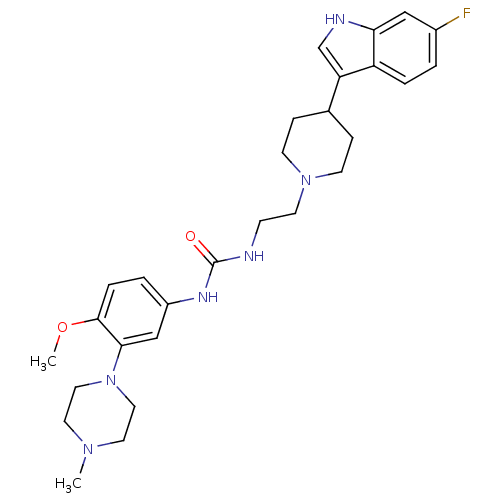 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-piperidin-1-yl]-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106701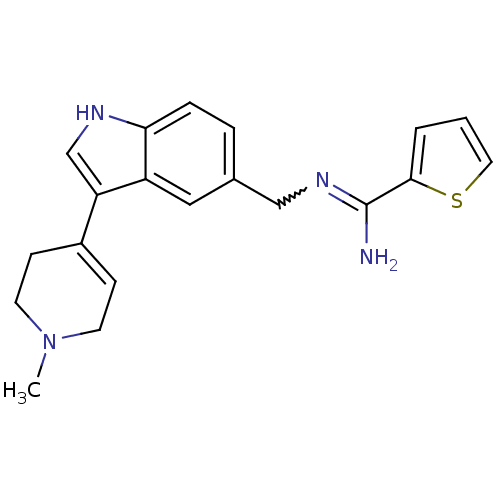 (CHEMBL1957352 | US8586620, 51) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086110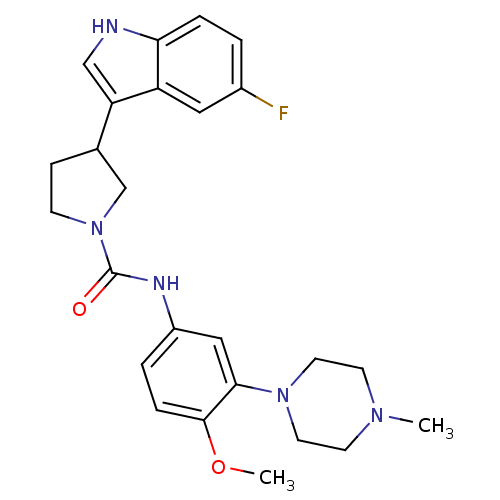 (3-(5-Fluoro-1H-indol-3-yl)-pyrrolidine-1-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086111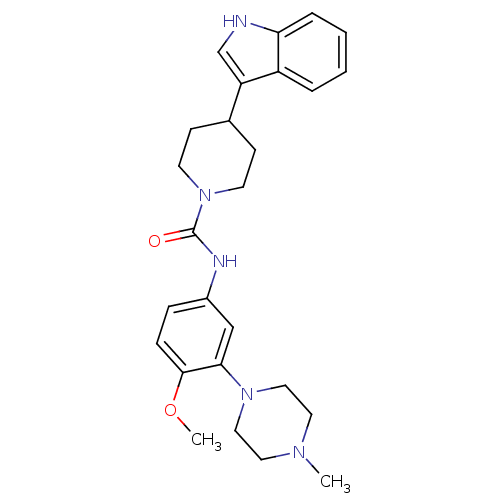 (4-(1H-Indol-3-yl)-piperidine-1-carboxylic acid [4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106700 (CHEMBL1957354 | US8586620, 47) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106704 (US8586620, 59) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086099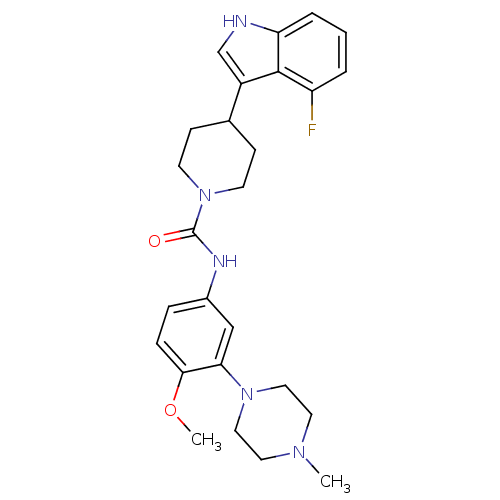 (4-(4-Fluoro-1H-indol-3-yl)-piperidine-1-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106720 (US8586620, 110) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106696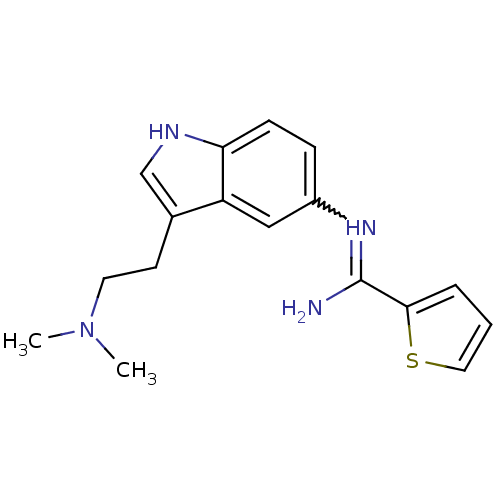 (US8586620, 56) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086101 (4-[4-(5-Fluoro-1H-indol-3-yl)-butyl]-piperazine-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086100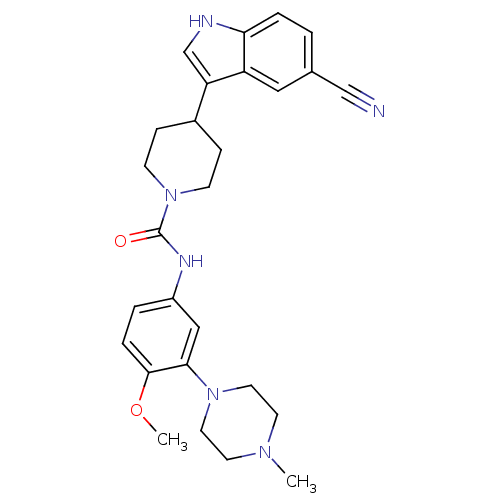 (4-(5-Cyano-1H-indol-3-yl)-piperidine-1-carboxylic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50081702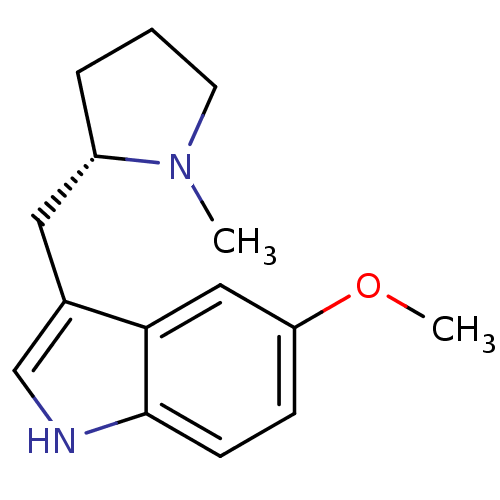 (5-Methoxy-3-((S)-1-methyl-pyrrolidin-2-ylmethyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc Curated by ChEMBL | Assay Description Binding affinity of the compound against 5-hydroxytryptamine 1D receptor | J Med Chem 35: 4503-5 (1992) BindingDB Entry DOI: 10.7270/Q29C6WC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086106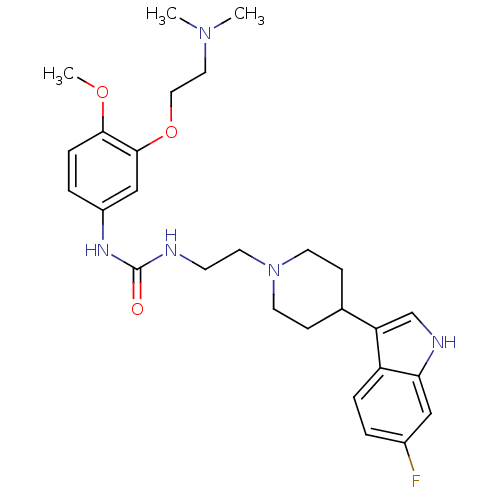 (1-[3-(2-Dimethylamino-ethoxy)-4-methoxy-phenyl]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106721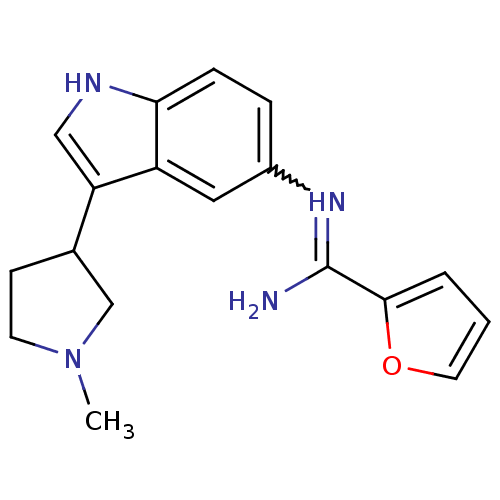 (US8586620, 111) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106697 (CHEMBL1957353 | US8586620, 43) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086103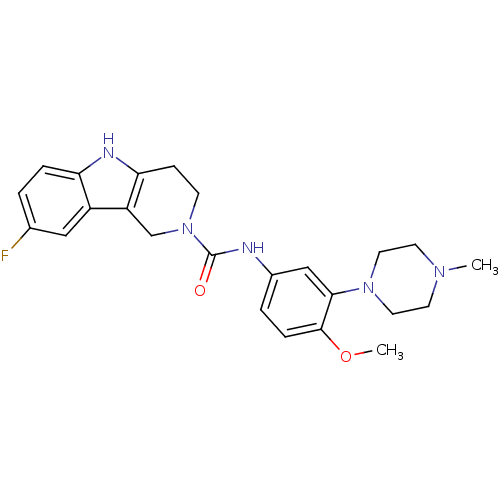 (8-Fluoro-1,3,4,5-tetrahydro-pyrido[4,3-b]indole-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 667 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106705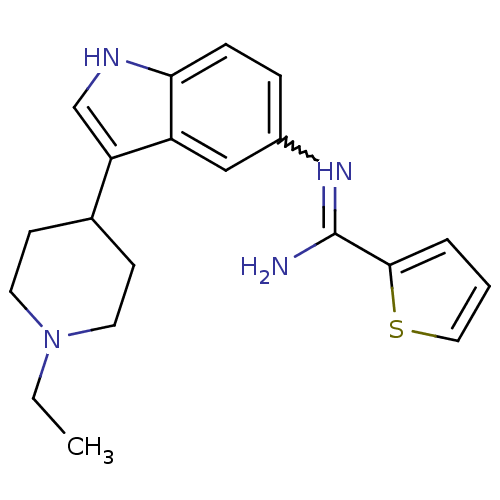 (US8586620, 62) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106703 (US8586620, 15) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086117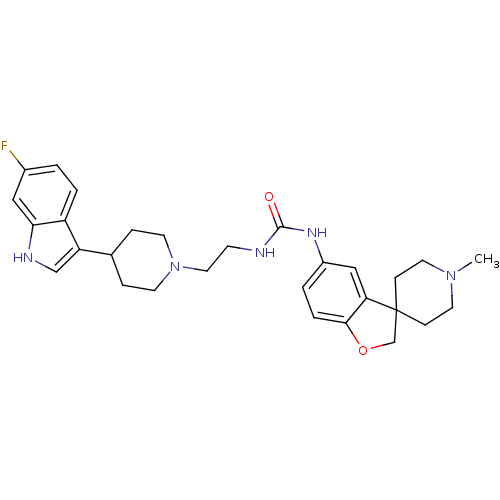 (1-{2-[4-(6-fluoro-1H-indol-3-yl)piperidin-1yl]ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086115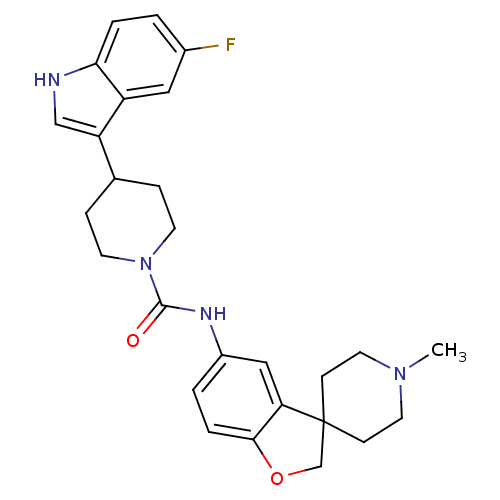 (5-[4-(5-fluoro-1H-3-indolyl)hexahydro-1-pyridinylc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086096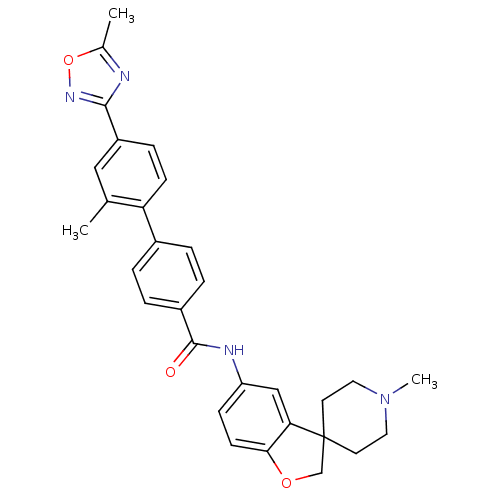 (2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50086113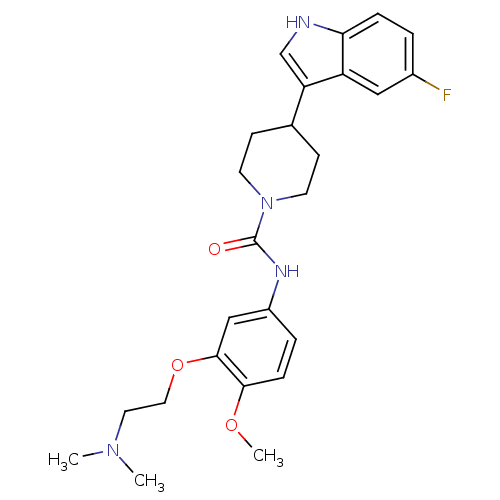 (4-(5-Fluoro-1H-indol-3-yl)-piperidine-1-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1D receptor in rat frontal cortex using [125I]-iodocyanopindolol as radio-ligand. | J Med Chem 43: 1149-57 (2000) BindingDB Entry DOI: 10.7270/Q2VD6XPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106717 (US8586620, 105) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of rat hydroxytryptamine 1D receptor | J Med Chem 47: 4684-92 (2004) Article DOI: 10.1021/jm040793q BindingDB Entry DOI: 10.7270/Q2513XPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||