

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50601551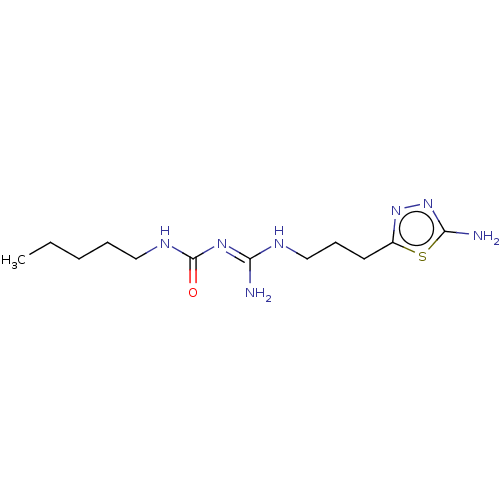 (CHEMBL5207281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50568740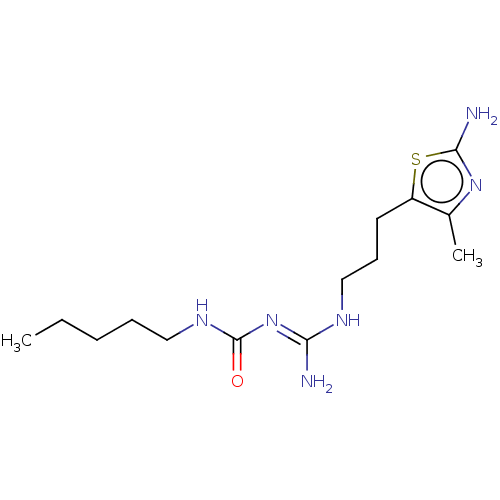 (CHEMBL4860528) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50601574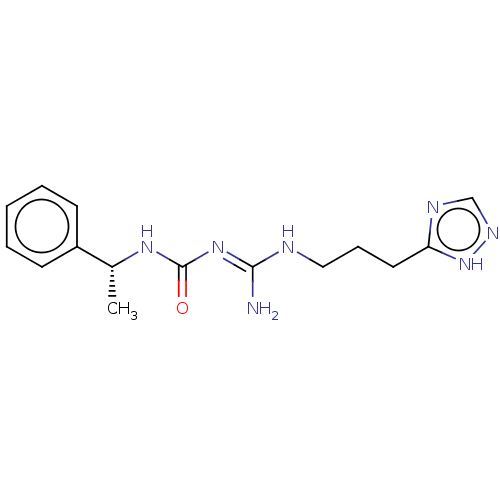 (CHEMBL5201074) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50601567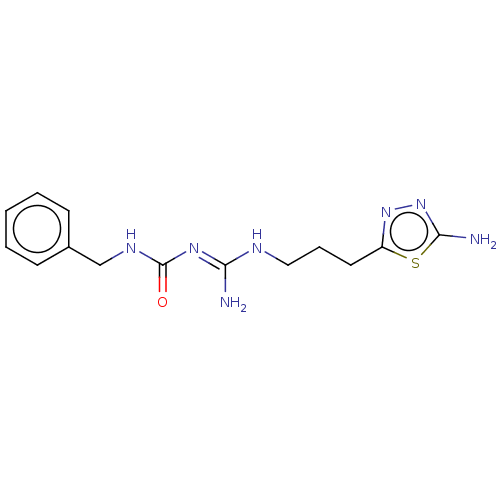 (CHEMBL5206565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00633 BindingDB Entry DOI: 10.7270/Q26M3BS8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined | J Med Chem 36: 1918-9 (1993) Article DOI: 10.1021/jm00065a015 BindingDB Entry DOI: 10.7270/Q2JS9SNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50035513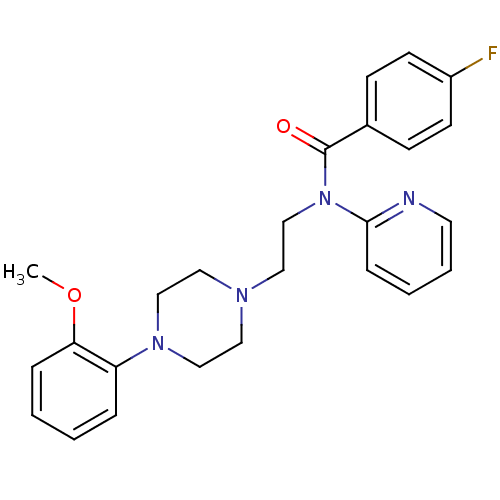 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00633 BindingDB Entry DOI: 10.7270/Q26M3BS8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50033383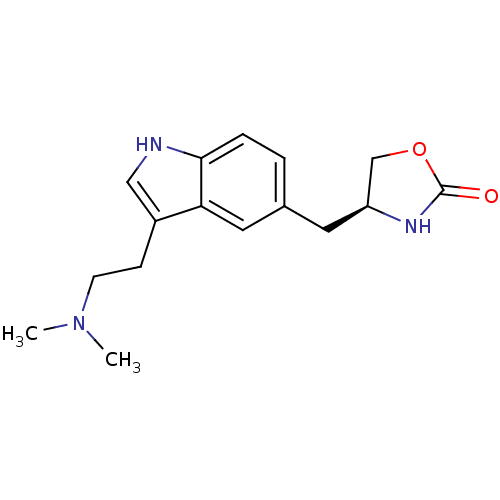 ((S)-4-((3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1 receptor using rabbit jugular vein assay in the absence of endothelium | J Med Chem 38: 3566-80 (1995) BindingDB Entry DOI: 10.7270/Q2GF0SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined | J Med Chem 36: 1918-9 (1993) Article DOI: 10.1021/jm00065a015 BindingDB Entry DOI: 10.7270/Q2JS9SNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50423462 (CHEMBL251835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem Lett 18: 979-82 (2008) Article DOI: 10.1016/j.bmcl.2007.12.030 BindingDB Entry DOI: 10.7270/Q2WQ053B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50423465 (ERGOLINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem Lett 18: 979-82 (2008) Article DOI: 10.1016/j.bmcl.2007.12.030 BindingDB Entry DOI: 10.7270/Q2WQ053B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50034355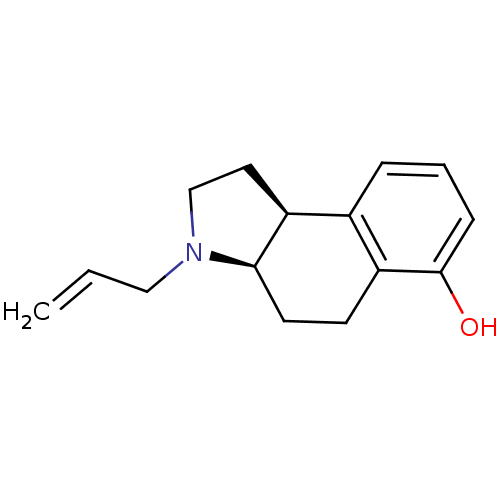 ((3aR,9bS)-3-Allyl-2,3,3a,4,5,9b-hexahydro-1H-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 52: 6107-25 (2009) Article DOI: 10.1021/jm901096y BindingDB Entry DOI: 10.7270/Q2J67HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50056445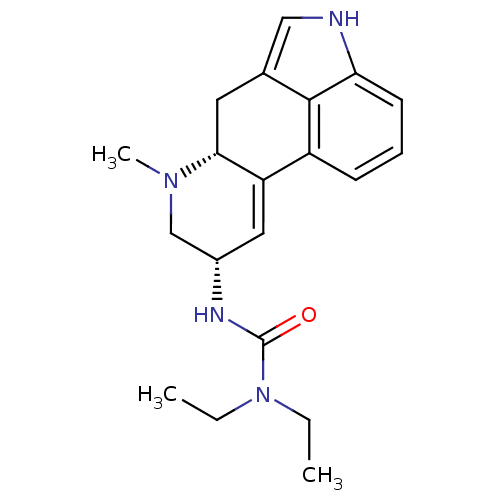 (1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor in piglet hippocampus using [3H]8-OH-DPAT | J Med Chem 36: 1918-9 (1993) Article DOI: 10.1021/jm00065a015 BindingDB Entry DOI: 10.7270/Q2JS9SNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM85099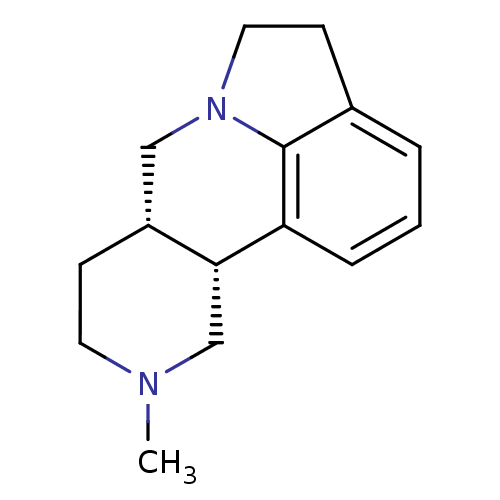 (CAS_141474-54-6 | SDZ SER 082 FUMARATE | SDZ SER-0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in pig frontal cortex membranes using [8H]-OH-DPAT | J Med Chem 38: 28-33 (1995) Article DOI: 10.1021/jm00001a007 BindingDB Entry DOI: 10.7270/Q2C2505V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor in piglet hippocampus using [3H]8-OH-DPAT | J Med Chem 36: 1918-9 (1993) Article DOI: 10.1021/jm00065a015 BindingDB Entry DOI: 10.7270/Q2JS9SNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM85099 (CAS_141474-54-6 | SDZ SER 082 FUMARATE | SDZ SER-0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in pig frontal cortex membranes using [8H]-OH-DPAT | J Med Chem 38: 28-33 (1995) Article DOI: 10.1021/jm00001a007 BindingDB Entry DOI: 10.7270/Q2C2505V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 52: 6107-25 (2009) Article DOI: 10.1021/jm901096y BindingDB Entry DOI: 10.7270/Q2J67HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523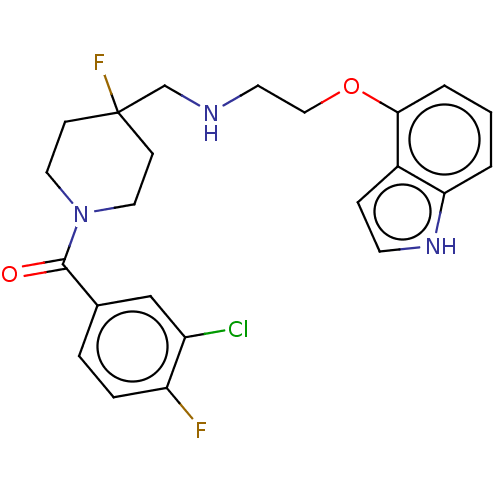 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537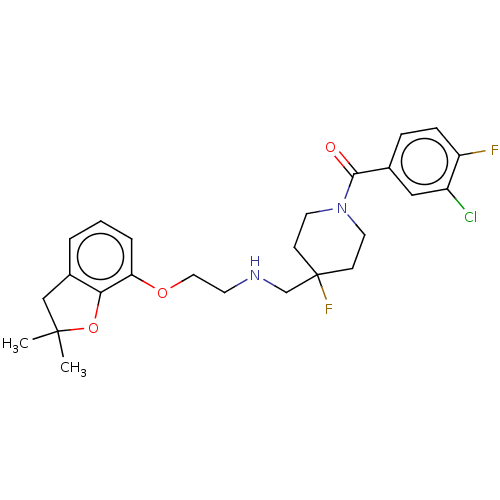 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433539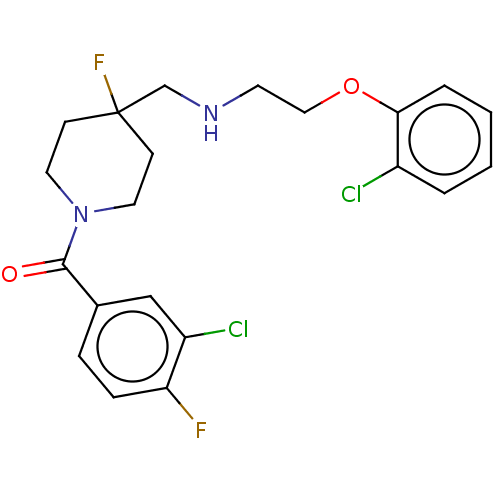 (US10562853, Compound 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50327601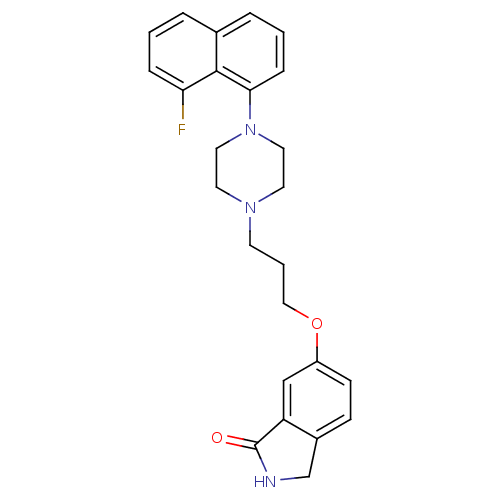 (6-(3-(4-(8-fluoronaphthalen-1-yl)piperazin-1-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells | Bioorg Med Chem Lett 20: 5666-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.023 BindingDB Entry DOI: 10.7270/Q2HH6K9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50555494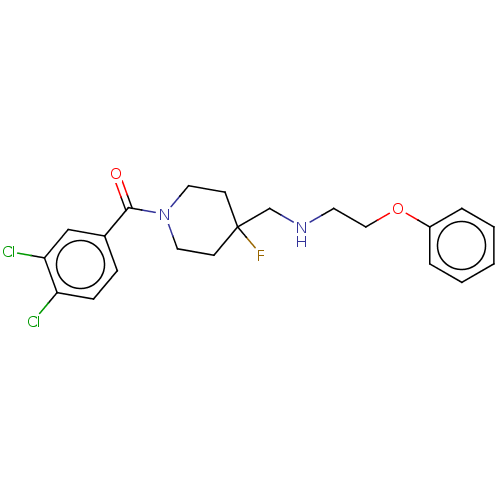 (CHEMBL4742112) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50327602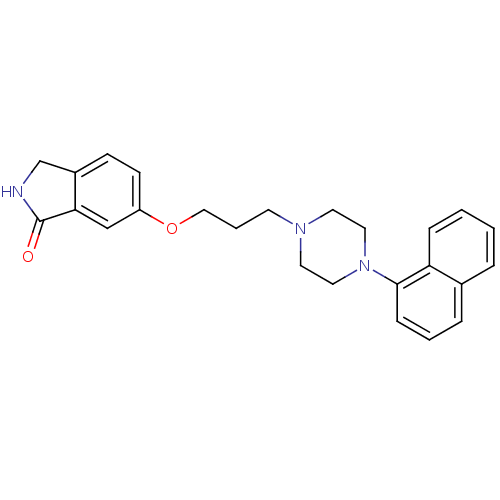 (6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)propoxy)is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells | Bioorg Med Chem Lett 20: 5666-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.023 BindingDB Entry DOI: 10.7270/Q2HH6K9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433538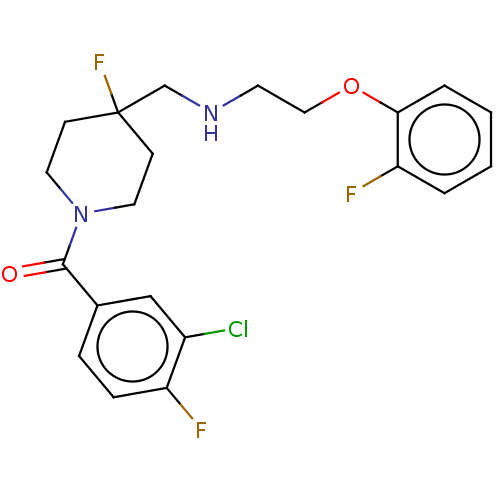 (US10562853, Compound 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433479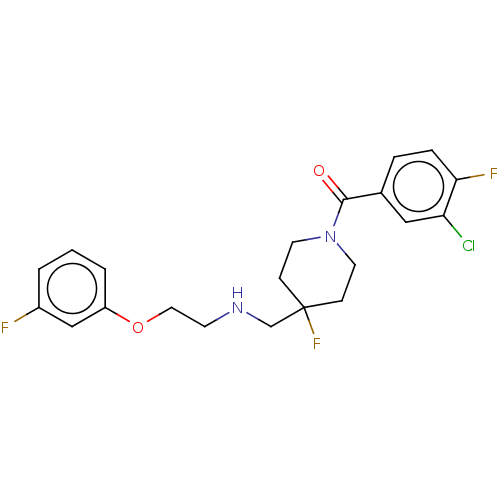 (US10562853, Compound 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50546855 (CHEMBL4752833) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433479 (US10562853, Compound 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50546856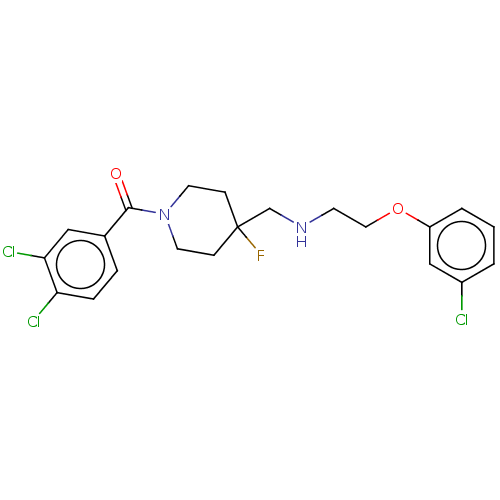 (CHEMBL4759759) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50274835 (CHEMBL511857 | trans-1-(4-(3-methoxyphenyl)cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institute and Karolinska University Hospital Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1A receptor expressed in human HeLa cell membranes assessed as stimulation of [35S]GTPgammaS binding after 20 mins by l... | Bioorg Med Chem 23: 4824-30 (2015) Article DOI: 10.1016/j.bmc.2015.05.042 BindingDB Entry DOI: 10.7270/Q21R6S8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433491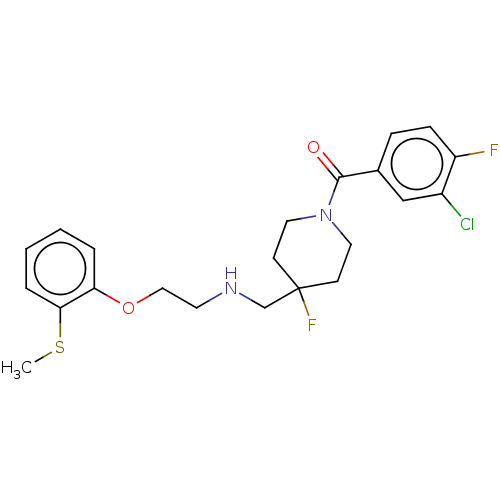 (US10562853, Compound 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433526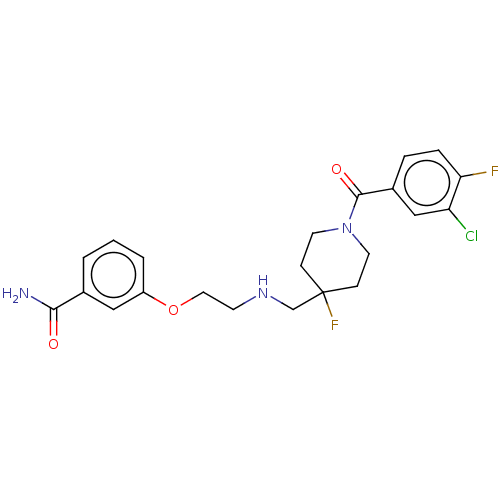 (US10562853, Compound 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50373901 (CHEMBL271987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in HEK293 cells after 120 mins | J Med Chem 51: 1492-5 (2008) Article DOI: 10.1021/jm7013919 BindingDB Entry DOI: 10.7270/Q2NG4RHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458528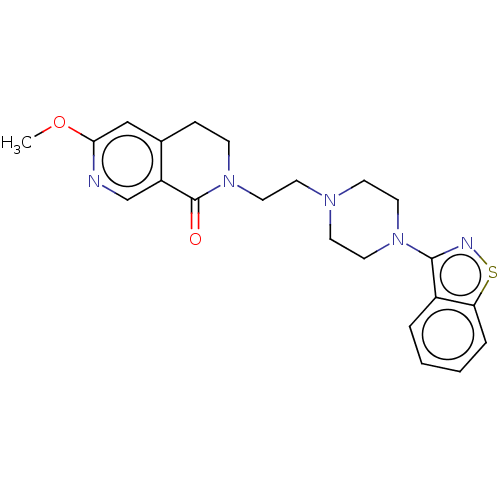 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458634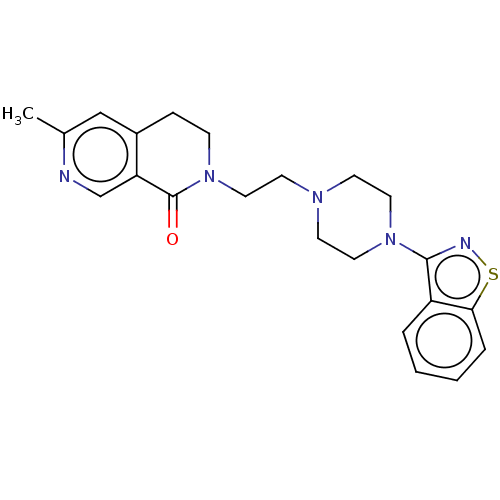 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433480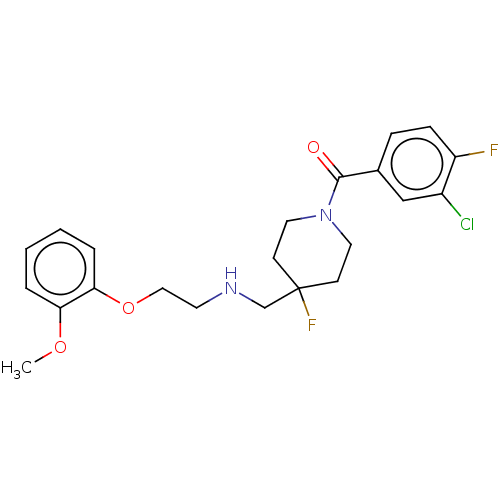 (US10562853, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433484 (US10562853, Compound 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433554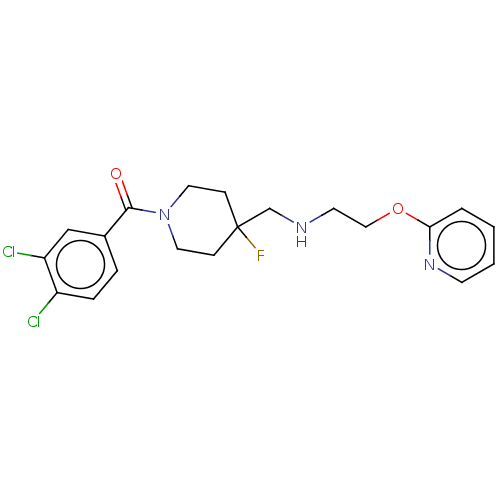 (US10562853, Compound 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433531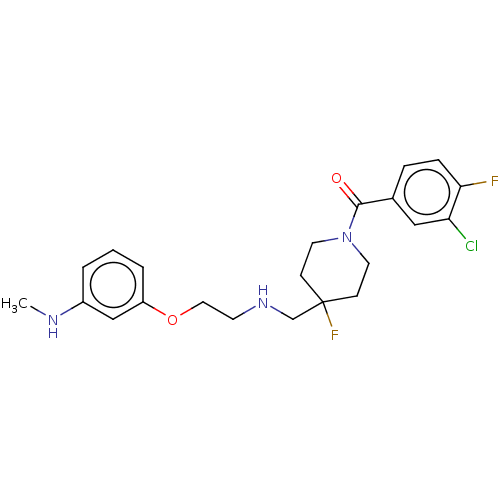 (US10562853, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433480 (US10562853, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50472582 (CHEMBL45422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00633 BindingDB Entry DOI: 10.7270/Q26M3BS8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50590640 (CHEMBL5177580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00633 BindingDB Entry DOI: 10.7270/Q26M3BS8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433478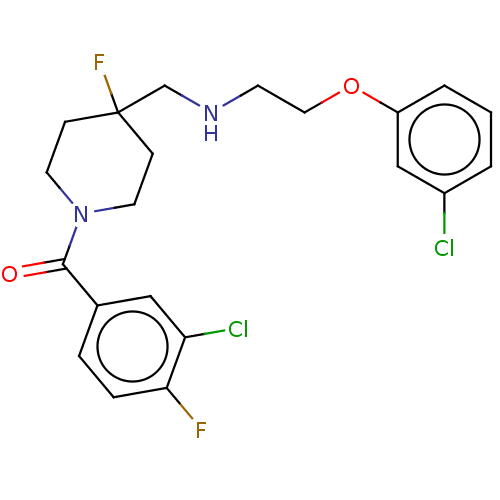 (US10562853, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50546857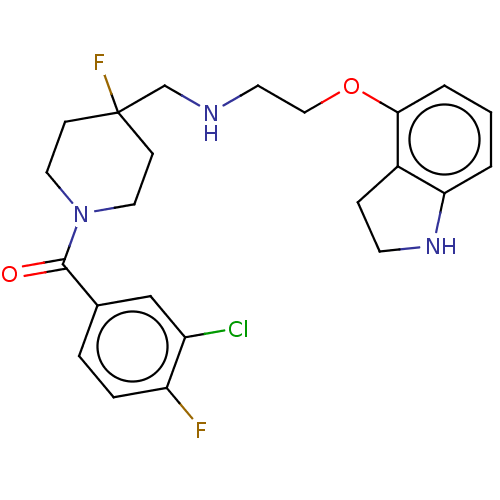 (CHEMBL4757755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50327603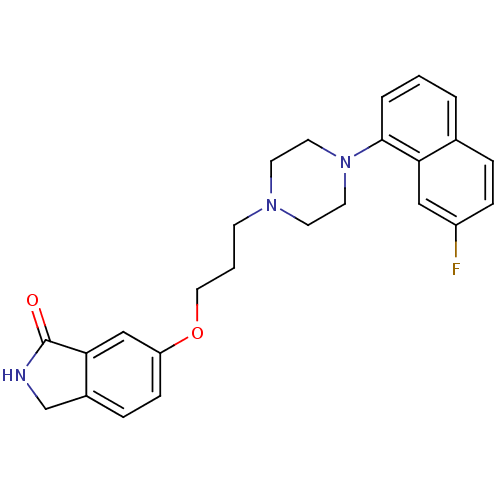 (6-(3-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells | Bioorg Med Chem Lett 20: 5666-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.023 BindingDB Entry DOI: 10.7270/Q2HH6K9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433478 (US10562853, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433549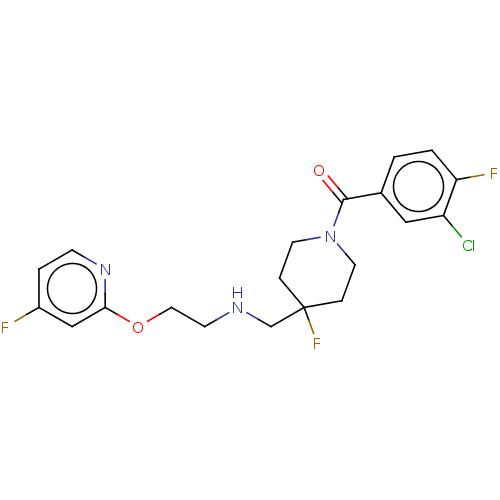 (US10562853, Compound 70) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50327604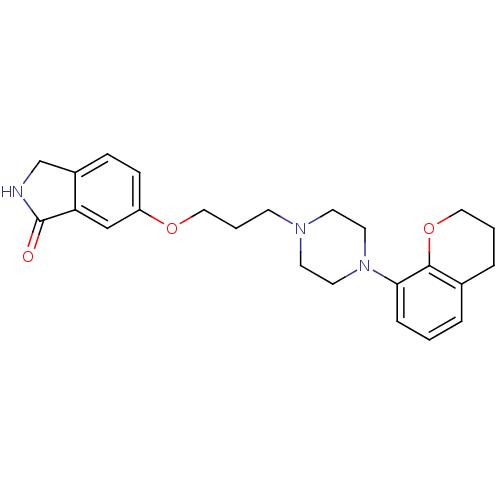 (6-(3-(4-(chroman-8-yl)piperazin-1-yl)propoxy)isoin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay | Bioorg Med Chem Lett 20: 5666-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.023 BindingDB Entry DOI: 10.7270/Q2HH6K9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50327605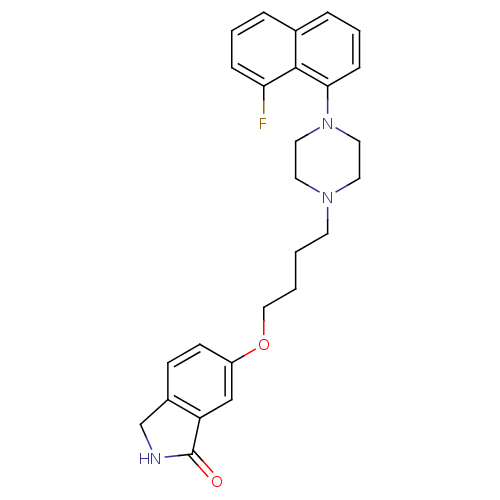 (6-(4-(4-(8-fluoronaphthalen-1-yl)piperazin-1-yl)bu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells | Bioorg Med Chem Lett 20: 5666-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.023 BindingDB Entry DOI: 10.7270/Q2HH6K9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433483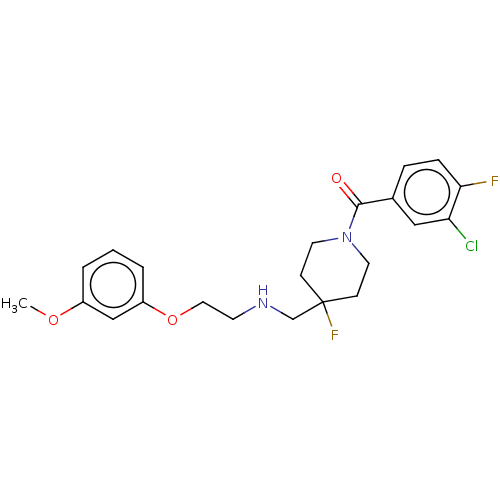 (US10562853, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8790 total ) | Next | Last >> |