

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM92326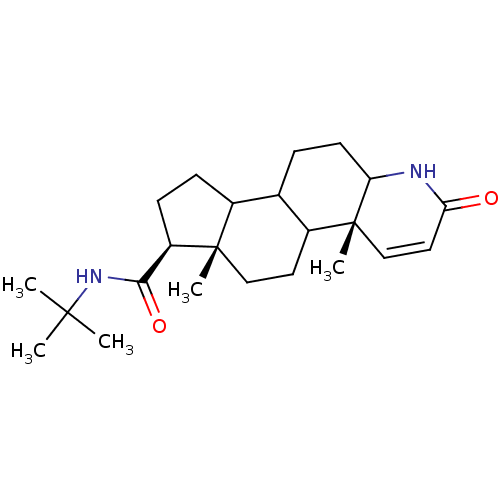 (Finasteride, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
National University of Mexico City | Assay Description The IC50 values for the synthesized steroids with human prostate 5alpha-reductase enzyme. | J Enzyme Inhib Med Chem 24: 655-62 (2009) Article DOI: 10.1080/14756360802323720 BindingDB Entry DOI: 10.7270/Q2ZK5F9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM92330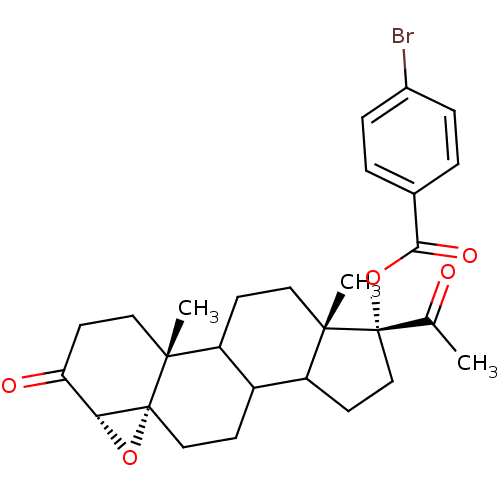 (Steroid, 10b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 6.5 | 37 |
National University of Mexico City | Assay Description The IC50 values for the synthesized steroids with human prostate 5alpha-reductase enzyme. | J Enzyme Inhib Med Chem 24: 655-62 (2009) Article DOI: 10.1080/14756360802323720 BindingDB Entry DOI: 10.7270/Q2ZK5F9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM92329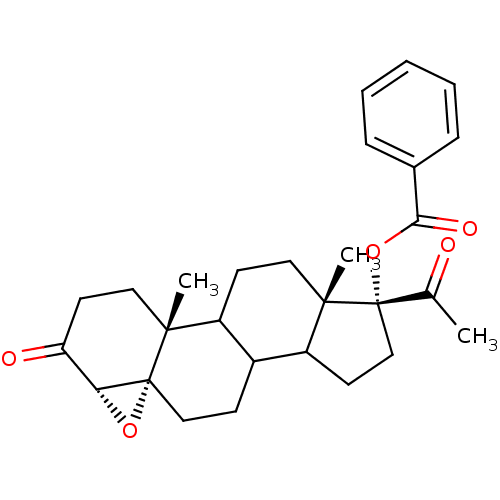 (Steroid, 10a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.5 | 37 |
National University of Mexico City | Assay Description The IC50 values for the synthesized steroids with human prostate 5alpha-reductase enzyme. | J Enzyme Inhib Med Chem 24: 655-62 (2009) Article DOI: 10.1080/14756360802323720 BindingDB Entry DOI: 10.7270/Q2ZK5F9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM92328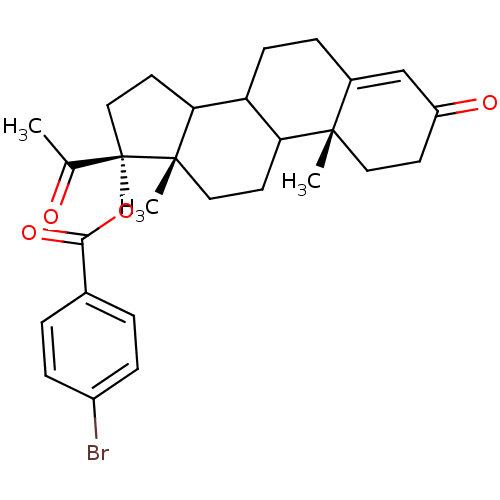 (Steroid, 9b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 6.5 | 37 |
National University of Mexico City | Assay Description The IC50 values for the synthesized steroids with human prostate 5alpha-reductase enzyme. | J Enzyme Inhib Med Chem 24: 655-62 (2009) Article DOI: 10.1080/14756360802323720 BindingDB Entry DOI: 10.7270/Q2ZK5F9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM92327 (Steroid, 9a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 6.5 | 37 |
National University of Mexico City | Assay Description The IC50 values for the synthesized steroids with human prostate 5alpha-reductase enzyme. | J Enzyme Inhib Med Chem 24: 655-62 (2009) Article DOI: 10.1080/14756360802323720 BindingDB Entry DOI: 10.7270/Q2ZK5F9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50180893 ((3-methyl-4-(4-phenoxybenzoyl)phenyl)acetic acid |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | 6.6 | n/a |
Saarland University Curated by ChEMBL | Assay Description Displacement of [3H]-androstenedione from 5alpha reductase1 in rat ventral prostrate homogenate at pH 6.6 | J Med Chem 49: 748-59 (2006) Article DOI: 10.1021/jm050728w BindingDB Entry DOI: 10.7270/Q2VQ3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243085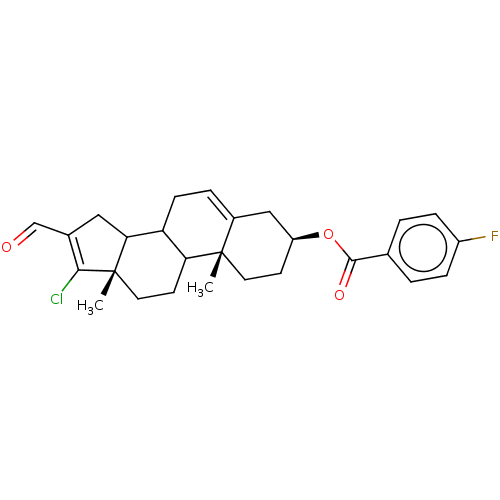 (17-Chloro-16-formylandrost-5,16-diene-3β-yl-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243086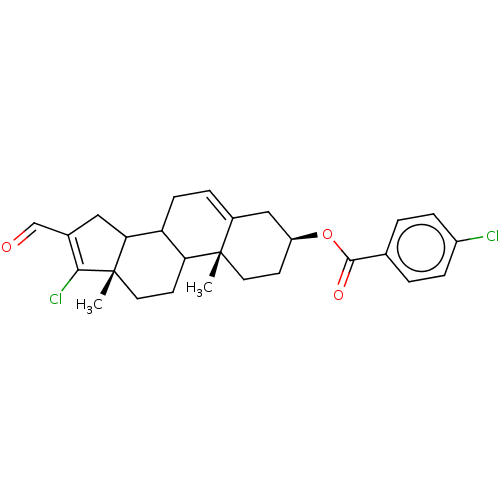 (17-Chloro-16-formylandrost-5,16-diene-3β-yl-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243087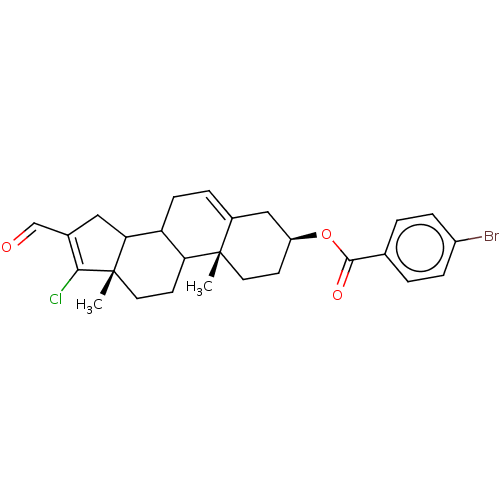 (17-Chloro-16-formylandros-5,16-diene-3β-yl-p-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243088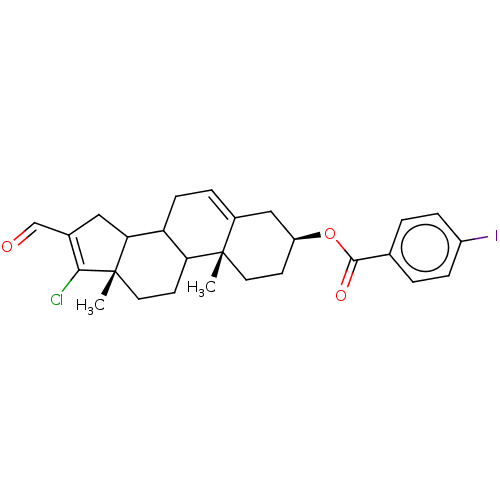 (17-Chloro-16-formylandrost-5,16-diene-3β-yl-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243089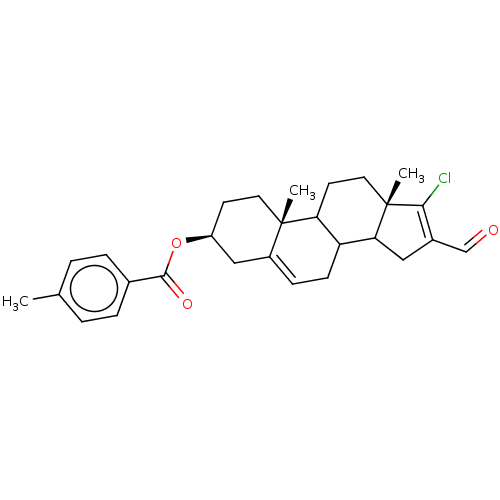 (17-Chloro-16-formylandrost-5,16-diene-3β-yl-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243084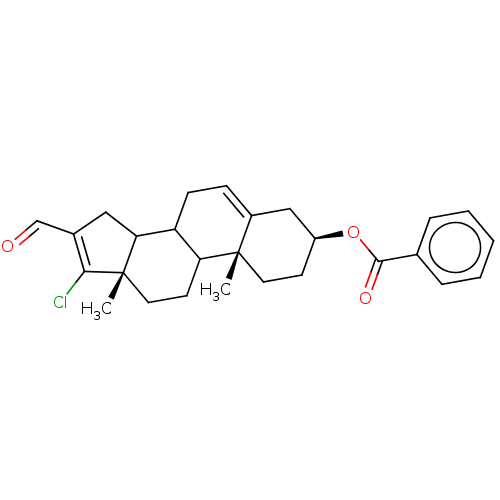 (17-Chloro-16-formylandrost-5,16-diene-3β-yl-b...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243083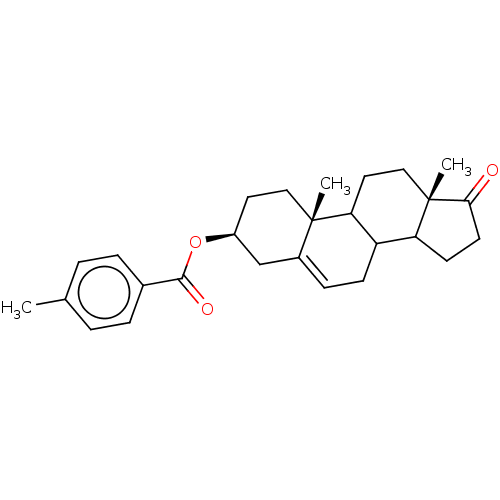 (17-Oxaandrost-5-ene-3β−yl-p-methylbenzo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243081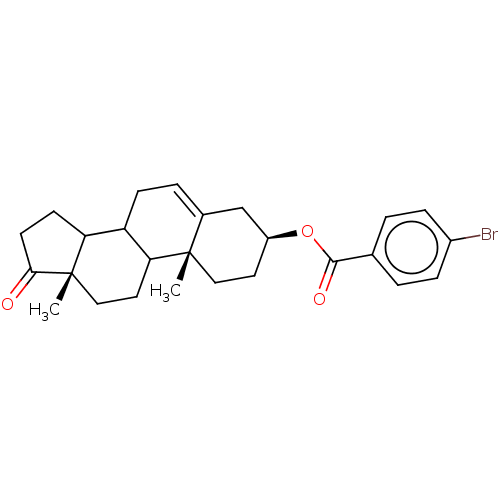 (17-Oxaandrost-5-ene-3β-yl-p-bromobenzoate (4)) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243080 (17-Oxaandrost-5-ene-3β-yl-p-chlorobenzoate (3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243079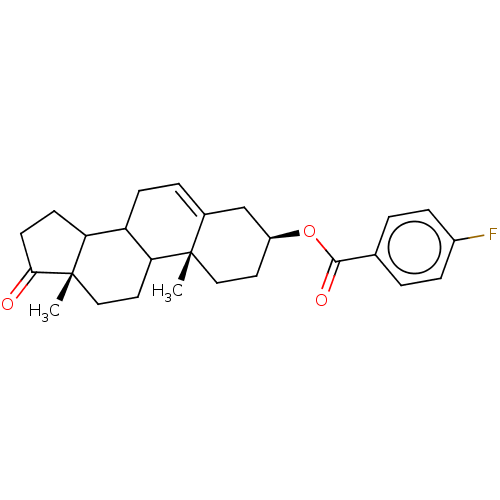 (17-Oxaandrost-5-ene-3β-yl-p-fluorobenzoate (2...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243078 (17-Oxaandrost-5-ene-3β-yl-benzoate (1)) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM243082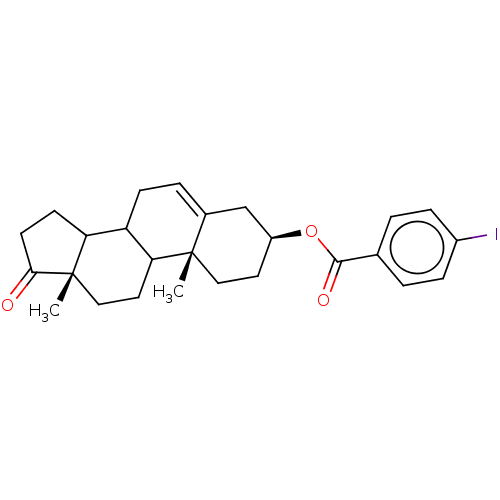 (17-Oxaandrost-5-ene-3β-yl-p-iodobenzoate (5)) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National University of Mexico City | Assay Description The activity of the 5α-R type 1 isozyme was determined by following the conversion of T to DHT at pH 7.5. 2 nM of [1,2,6,7 3H] T, and different ... | J Enzyme Inhib Med Chem 28: 1247-54 (2013) Article DOI: 10.3109/14756366.2012.729827 BindingDB Entry DOI: 10.7270/Q2SX6C4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031895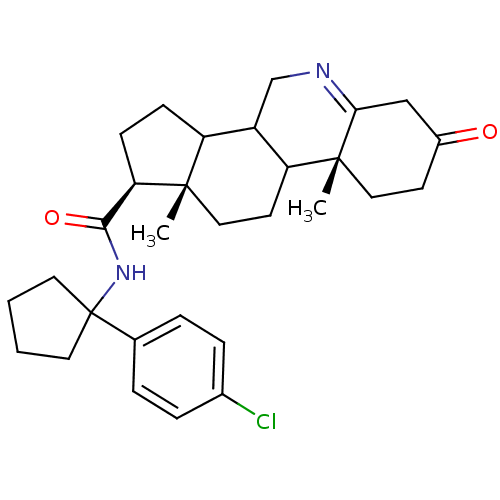 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50039257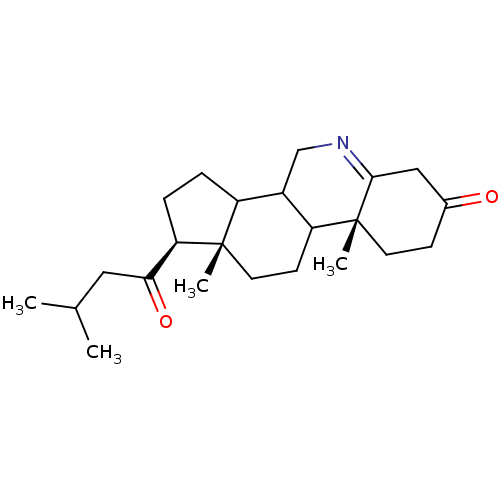 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031877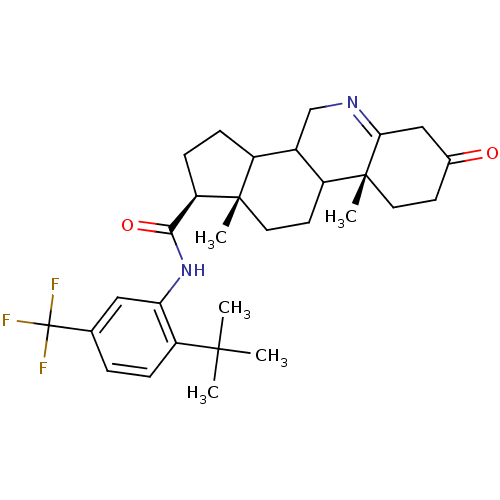 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031895 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031874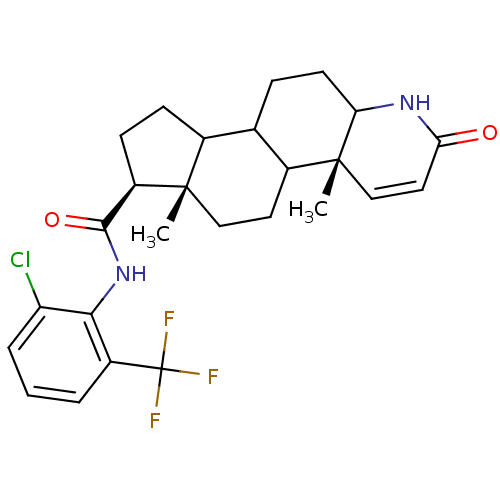 ((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Binding affinity to recombinant human Steroid 5-alpha-reductase type I was evaluated | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031877 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031883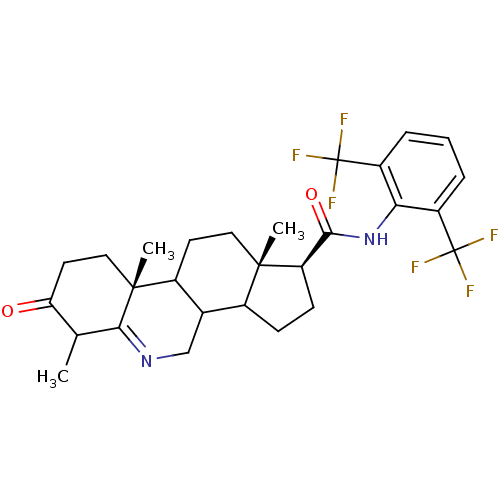 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057500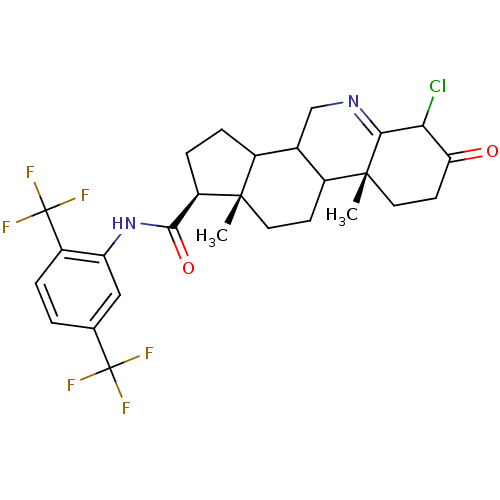 ((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for human 5 alpha reductase 1 isozyme | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50057484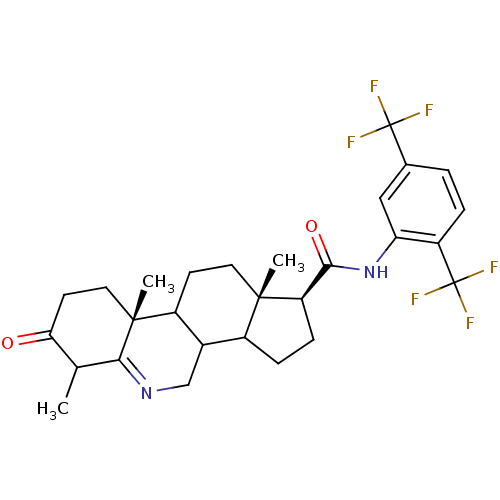 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for human 5 alpha reductase 1 isozyme | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50407405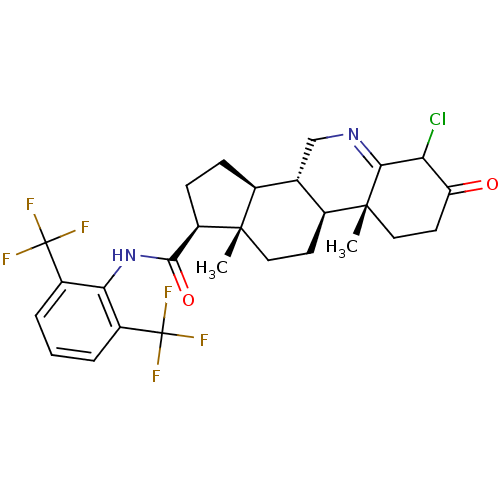 (CHEMBL2115222) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50043604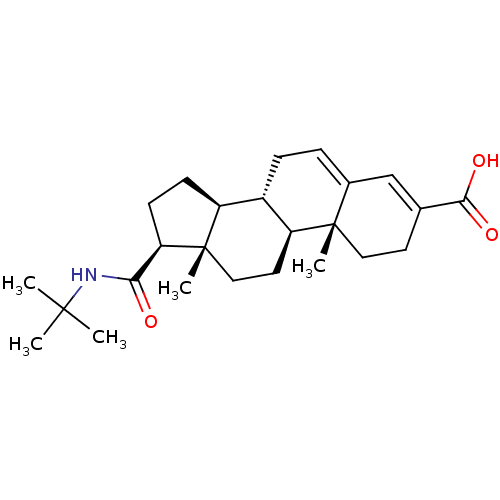 ((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50407405 (CHEMBL2115222) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50043604 ((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 4: 2327-2330 (1994) Article DOI: 10.1016/0960-894X(94)85034-8 BindingDB Entry DOI: 10.7270/Q25Q4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031896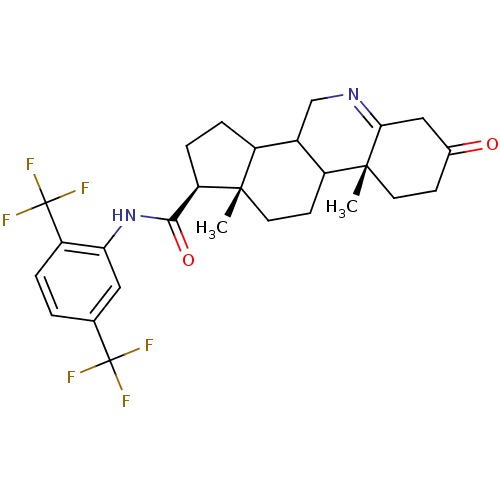 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031889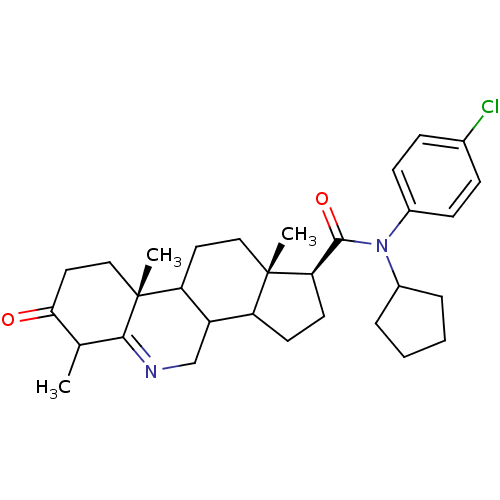 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031889 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for human 5 alpha reductase 1 isozyme | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368883 (CHEMBL1159458) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50403606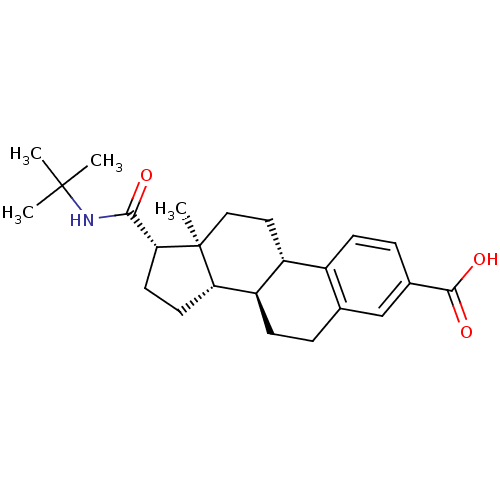 (CHEMBL1627951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031878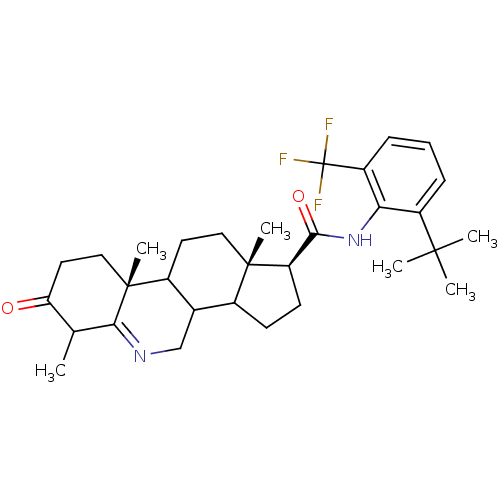 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031897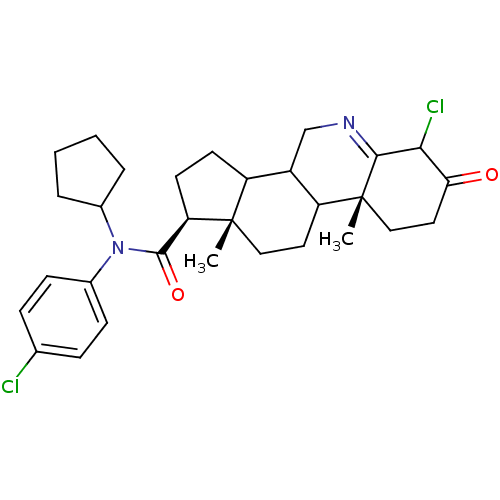 ((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031897 ((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Binding affinity for human 5 alpha reductase 1 isozyme | J Med Chem 40: 1293-315 (1997) Article DOI: 10.1021/jm960697s BindingDB Entry DOI: 10.7270/Q2W096MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50403324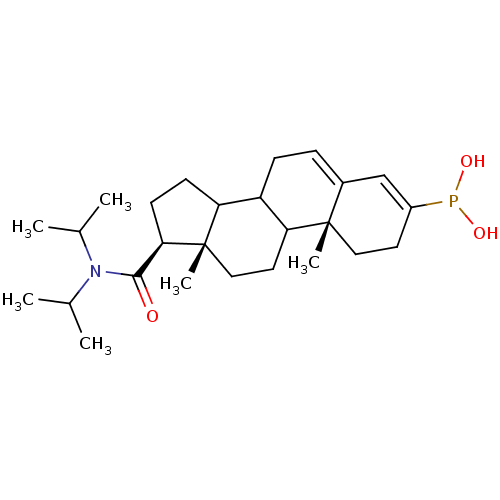 (CHEMBL78060) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 4: 2327-2330 (1994) Article DOI: 10.1016/0960-894X(94)85034-8 BindingDB Entry DOI: 10.7270/Q25Q4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50039285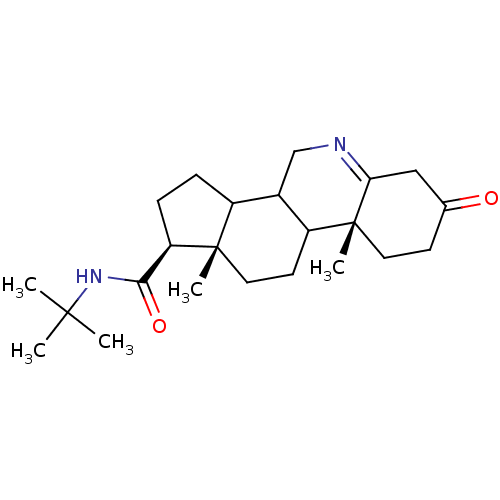 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039276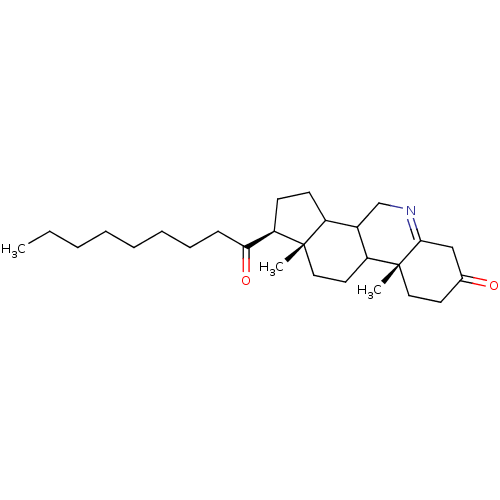 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-nonanoyl-1,2,3,3a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031896 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039277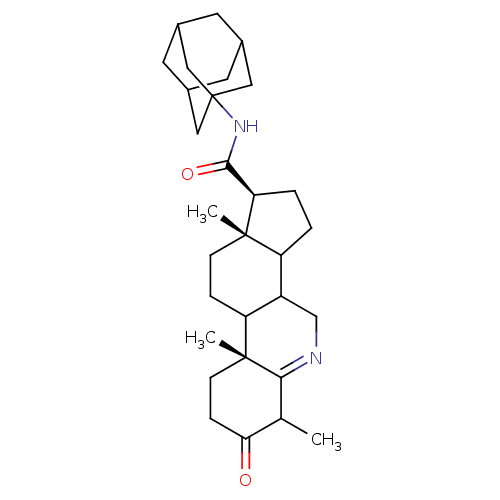 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031903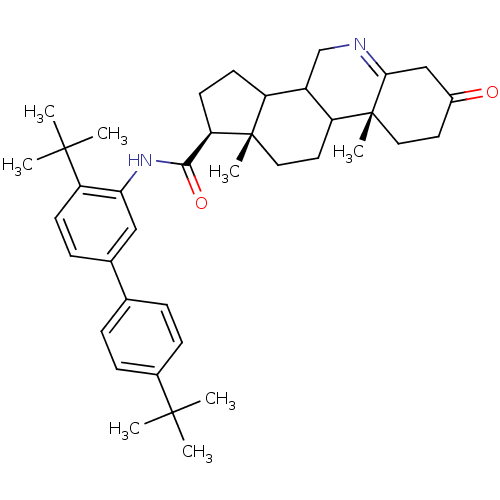 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50403610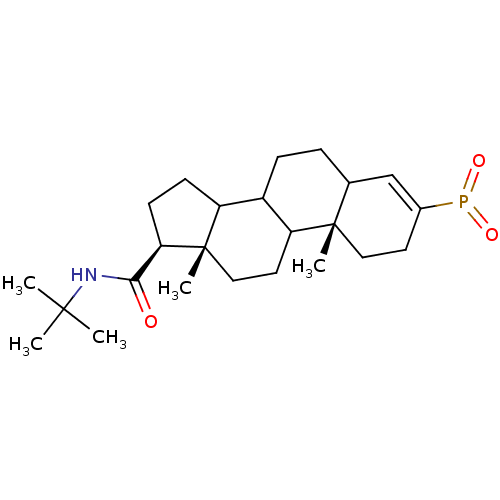 (CHEMBL143220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031879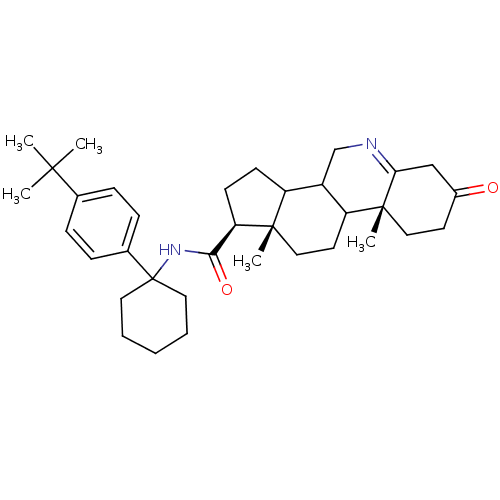 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 | Bioorg Med Chem Lett 4: 2327-2330 (1994) Article DOI: 10.1016/0960-894X(94)85034-8 BindingDB Entry DOI: 10.7270/Q25Q4X8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50213061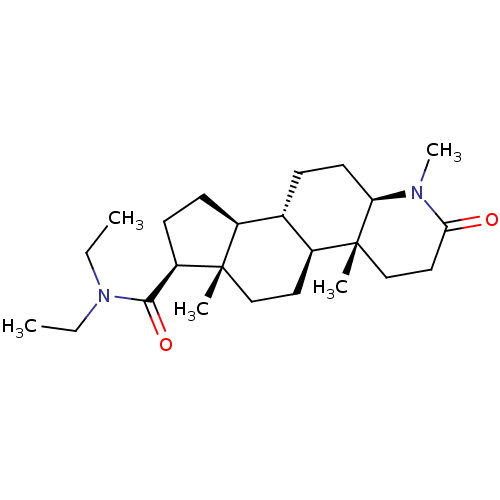 (CHEMBL2298601) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of type-2 human steroid 5-alpha-reductase. | Bioorg Med Chem Lett 6: 481-484 (1996) Article DOI: 10.1016/0960-894X(96)00054-6 BindingDB Entry DOI: 10.7270/Q2BZ661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1245 total ) | Next | Last >> |