

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120748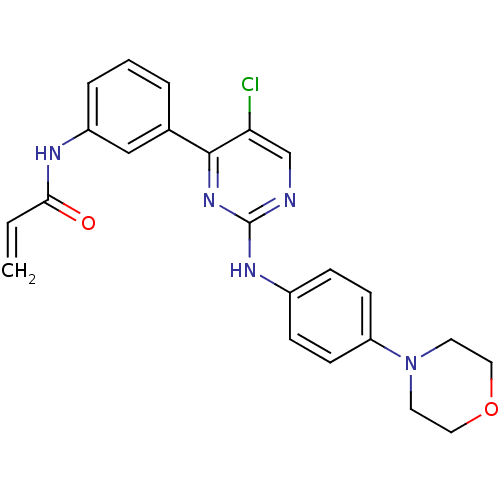 (US8703767, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120741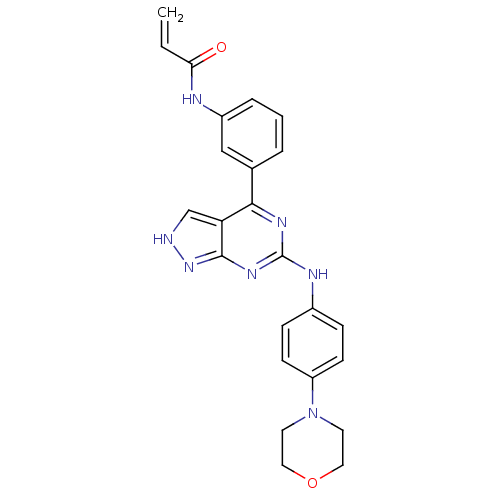 (US8703767, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120748 (US8703767, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120741 (US8703767, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120743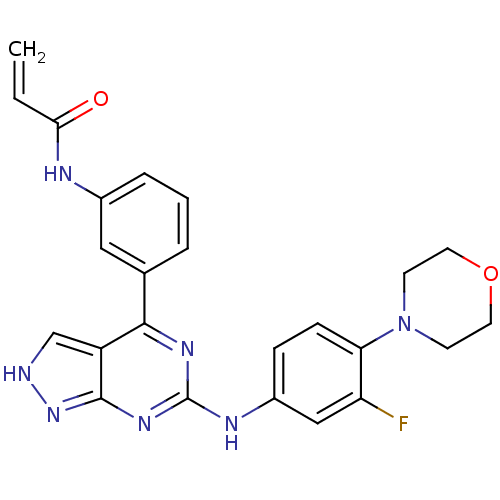 (US8703767, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120742 (US8703767, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120743 (US8703767, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120744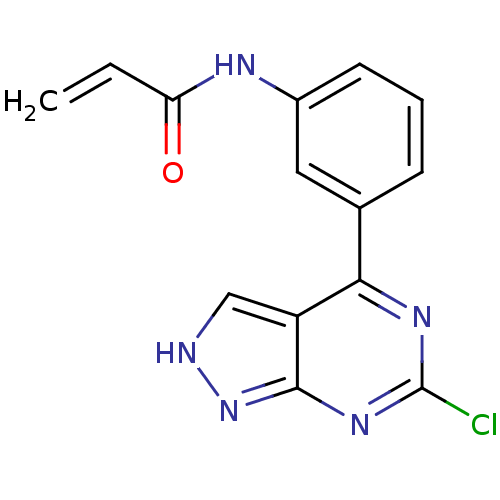 (US8703767, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120747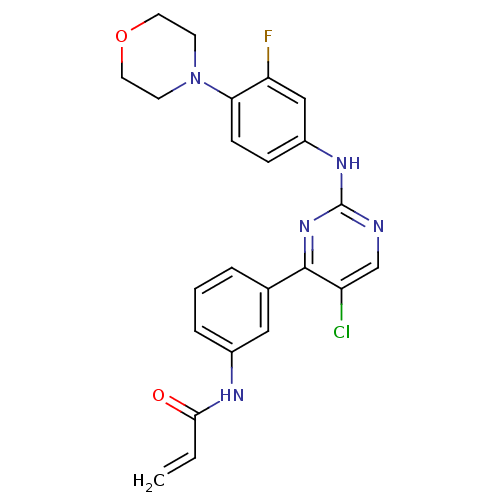 (US8703767, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120747 (US8703767, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120742 (US8703767, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120752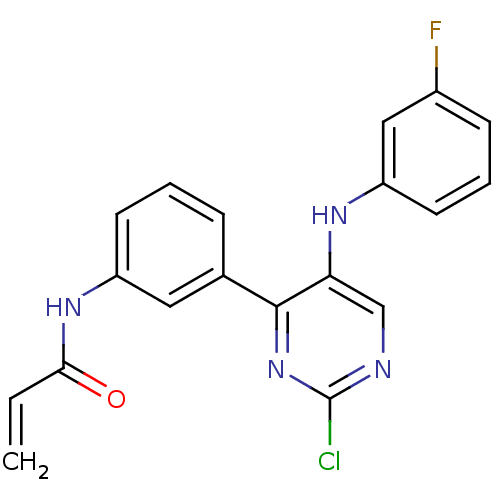 (US8703767, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 829 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120749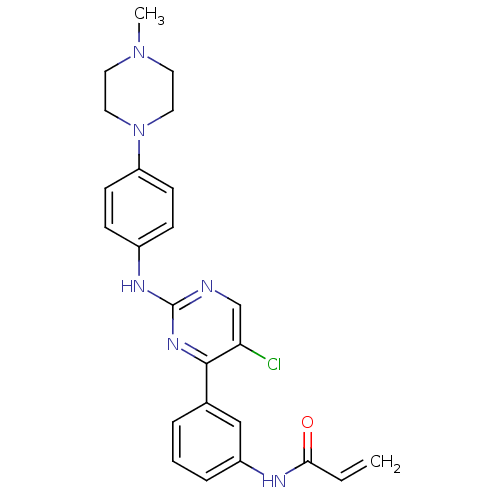 (US8703767, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120745 (US8703767, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120744 (US8703767, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120750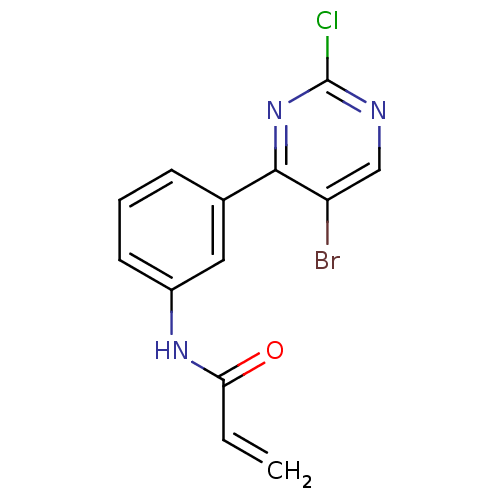 (US8703767, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120751 (US8703767, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120746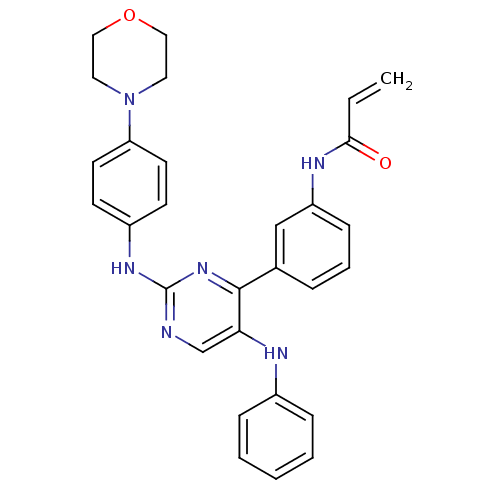 (US8703767, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||