

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088846 (CHEMBL353678 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-e...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088844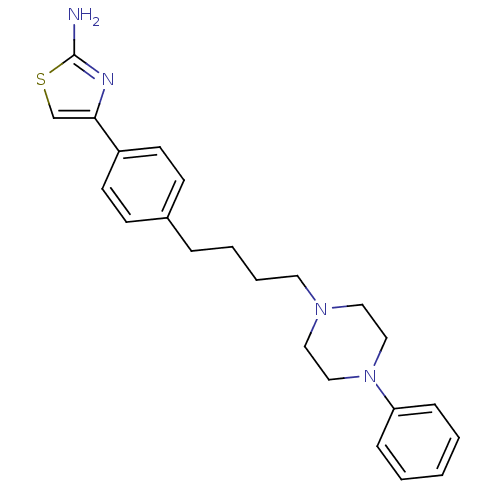 (4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088839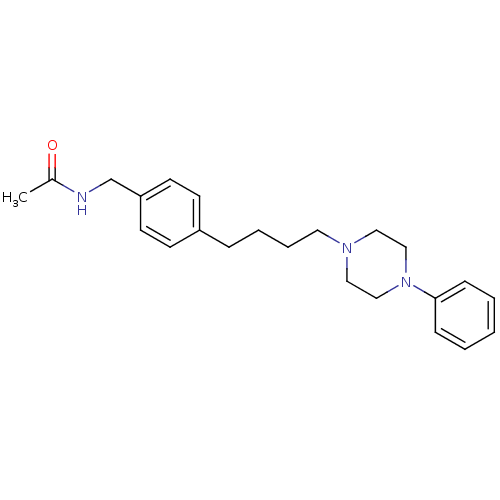 (CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human trypsin. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088844 (4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088839 (CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088841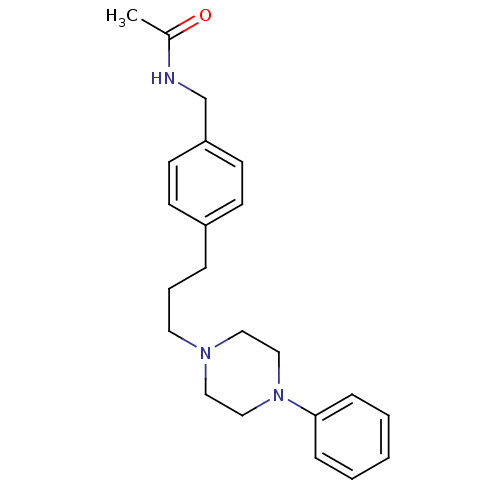 (CHEMBL352617 | N-{4-[3-(4-Phenyl-piperazin-1-yl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088844 (4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of class C beta-lactamase derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088841 (CHEMBL352617 | N-{4-[3-(4-Phenyl-piperazin-1-yl)-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088844 (4-{4-[4-(4-Phenyl-piperazin-1-yl)-butyl]-phenyl}-t...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of GC1 extended spectrum class C beta-lactamase | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088839 (CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of GC1 extended spectrum class C beta-lactamase | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088839 (CHEMBL169702 | N-{4-[4-(4-Phenyl-piperazin-1-yl)-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088846 (CHEMBL353678 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human trypsin. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088846 (CHEMBL353678 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-e...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088845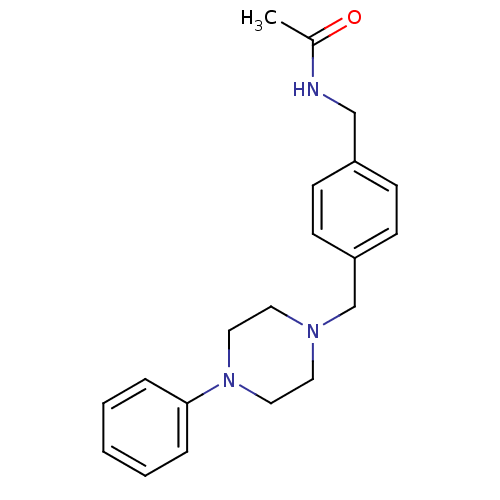 (CHEMBL170307 | N-[4-(4-Phenyl-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088841 (CHEMBL352617 | N-{4-[3-(4-Phenyl-piperazin-1-yl)-p...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of GC1 extended spectrum class C beta-lactamase | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088846 (CHEMBL353678 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088841 (CHEMBL352617 | N-{4-[3-(4-Phenyl-piperazin-1-yl)-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088847 (CHEMBL171345 | N-{4-[4-(4-Chloro-phenyl)-piperazin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of class C beta-lactamase derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088843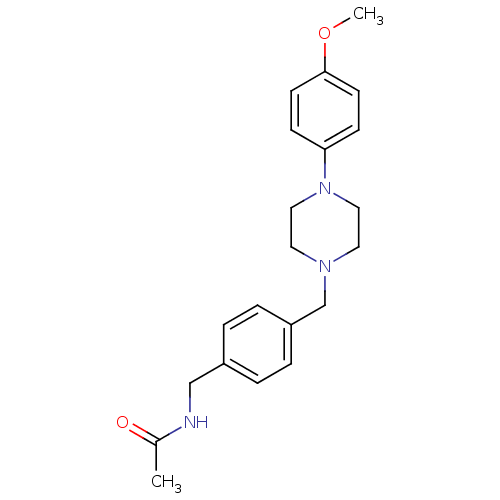 (CHEMBL171115 | N-{4-[4-(4-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088845 (CHEMBL170307 | N-[4-(4-Phenyl-piperazin-1-ylmethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088845 (CHEMBL170307 | N-[4-(4-Phenyl-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human plasmin. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088840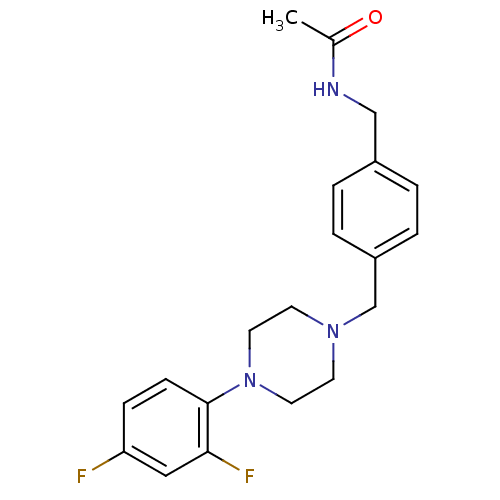 (CHEMBL352576 | N-{4-[4-(2,4-Difluoro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088840 (CHEMBL352576 | N-{4-[4-(2,4-Difluoro-phenyl)-piper...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088842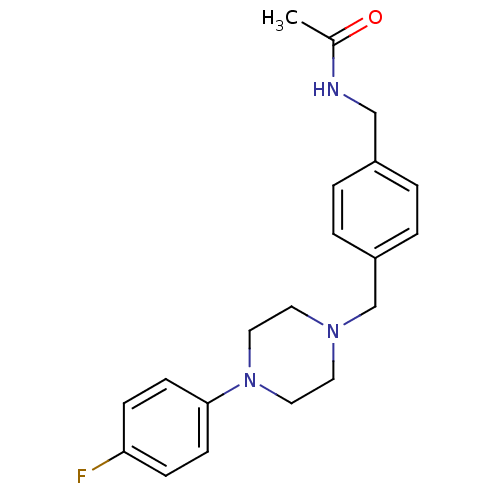 (CHEMBL171052 | N-{4-[4-(4-Fluoro-phenyl)-piperazin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088843 (CHEMBL171115 | N-{4-[4-(4-Methoxy-phenyl)-piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088847 (CHEMBL171345 | N-{4-[4-(4-Chloro-phenyl)-piperazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088845 (CHEMBL170307 | N-[4-(4-Phenyl-piperazin-1-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of GC1 extended spectrum class C beta-lactamase | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088842 (CHEMBL171052 | N-{4-[4-(4-Fluoro-phenyl)-piperazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50088843 (CHEMBL171115 | N-{4-[4-(4-Methoxy-phenyl)-piperazi...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088840 (CHEMBL352576 | N-{4-[4-(2,4-Difluoro-phenyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088847 (CHEMBL171345 | N-{4-[4-(4-Chloro-phenyl)-piperazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50088842 (CHEMBL171052 | N-{4-[4-(4-Fluoro-phenyl)-piperazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088840 (CHEMBL352576 | N-{4-[4-(2,4-Difluoro-phenyl)-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50088847 (CHEMBL171345 | N-{4-[4-(4-Chloro-phenyl)-piperazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of class C beta-lactamase derived from Enterobacter cloacae P99 | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088843 (CHEMBL171115 | N-{4-[4-(4-Methoxy-phenyl)-piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50088842 (CHEMBL171052 | N-{4-[4-(4-Fluoro-phenyl)-piperazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 10: 875-9 (2000) BindingDB Entry DOI: 10.7270/Q2CZ36C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||