

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Rattus norvegicus) | BDBM50126158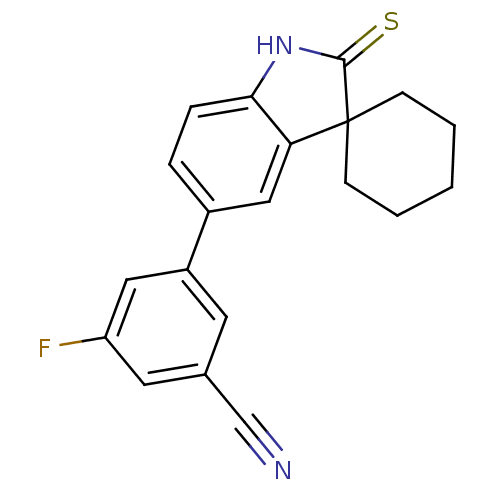 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory progestational activity on oral administration in uterine C3 model | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of 3 nM [3H]R5020 binding to progesterone receptor in human T47D cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50126158 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of 3 nM [3H]R5020 binding to progesterone receptor in human T47D cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50126158 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity against glucocorticoid receptor in human lung A549 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50121180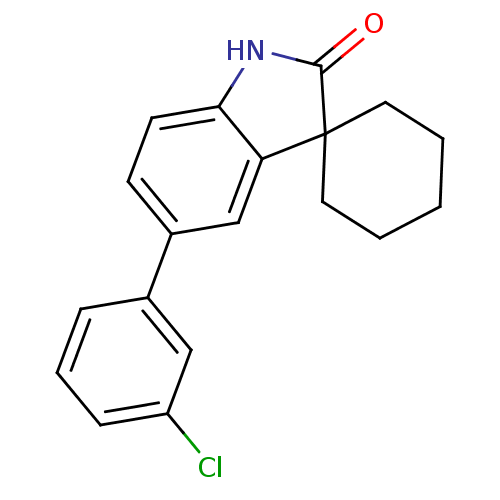 (5'-(3-chlorophenyl)spiro[cyclohexane-1,3'-indol]-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity against progesterone receptor induced alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 67.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory progestational activity on oral administration in uterine C3 model | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM50404222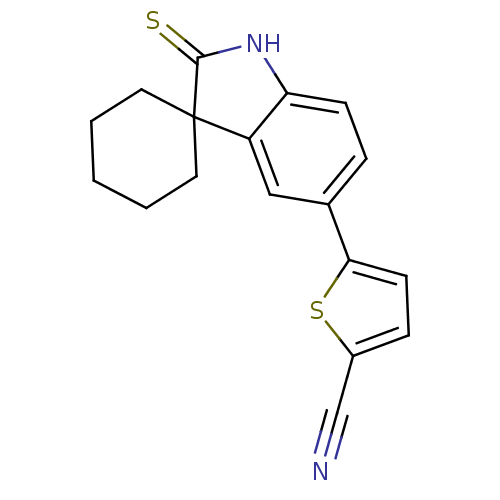 (CHEMBL417475) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concnetration against Androgen receptor in mouse fibroblast L929 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM50375837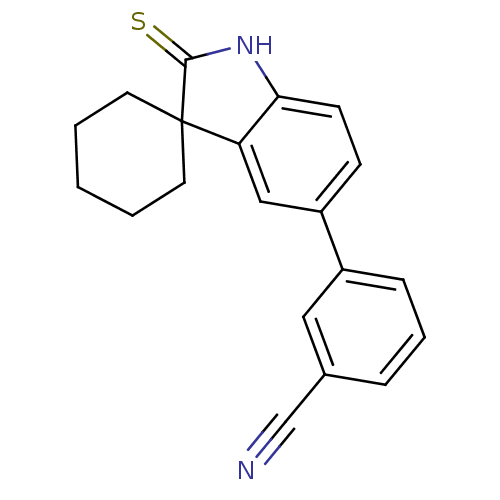 (CHEMBL270976) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 942 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM50404229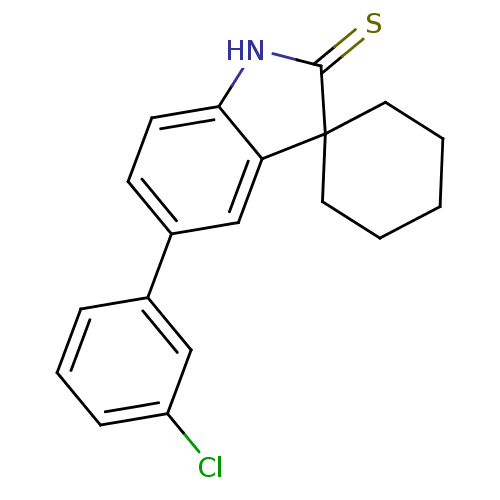 (CHEMBL24089) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory progestational activity on oral administration in uterine C3 model | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50126158 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity against Androgen receptor in mouse fibroblast L929 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404230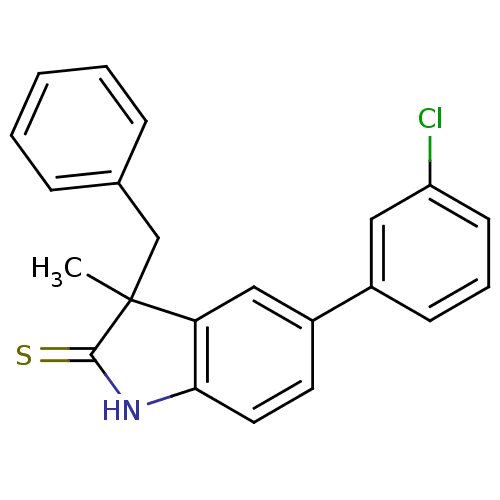 (CHEMBL26755) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404212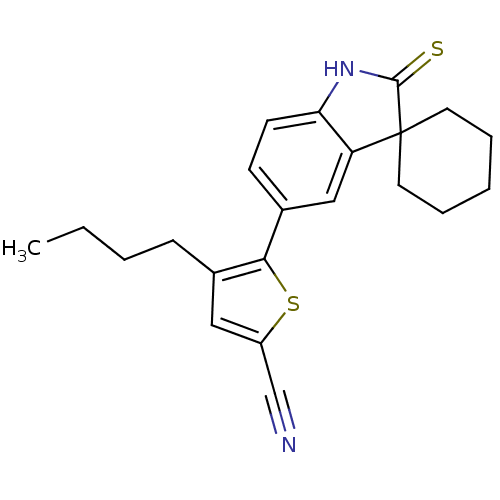 (CHEMBL27052) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404229 (CHEMBL24089) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404223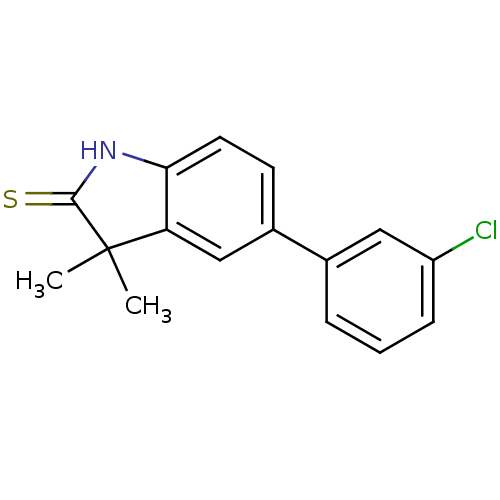 (CHEMBL280805) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404234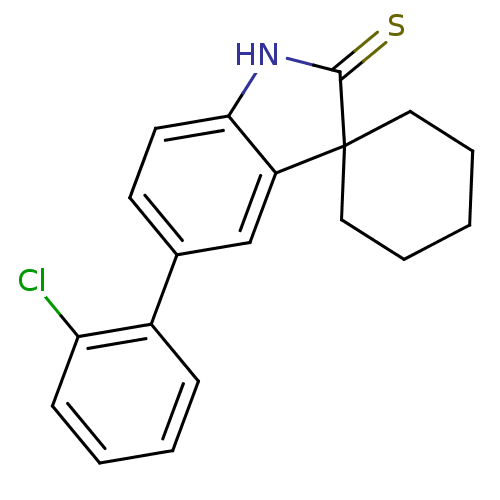 (CHEMBL25061) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404211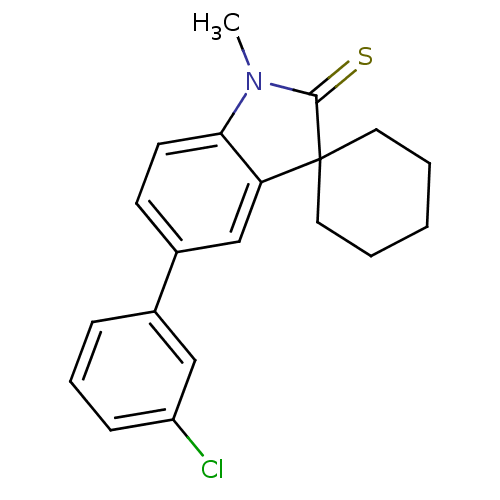 (CHEMBL25182) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50126158 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration against Androgen receptor in mouse fibroblast L929 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404219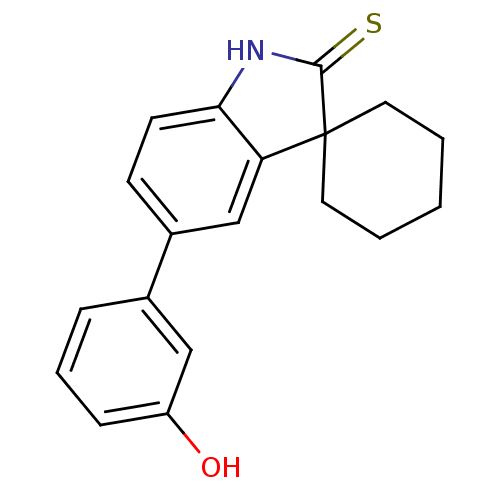 (CHEMBL283337) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404224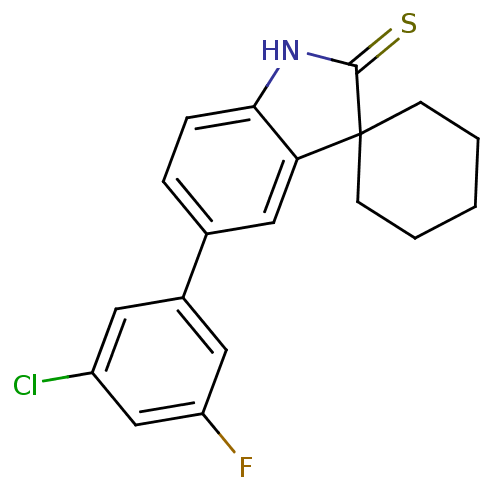 (CHEMBL27379) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404235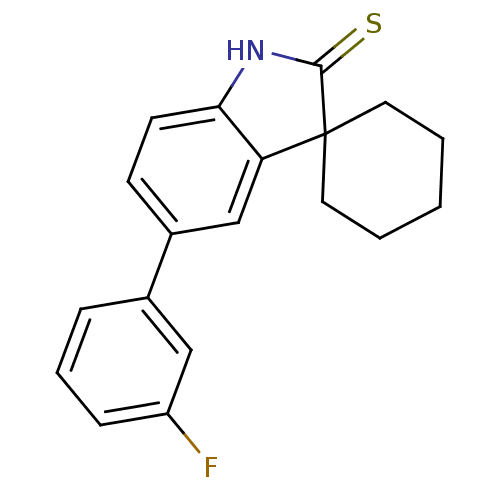 (CHEMBL25021) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404215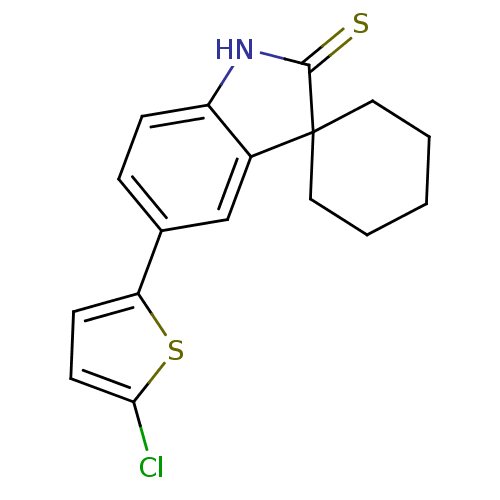 (CHEMBL27818) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration against glucocorticoid receptor in human lung A549 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404225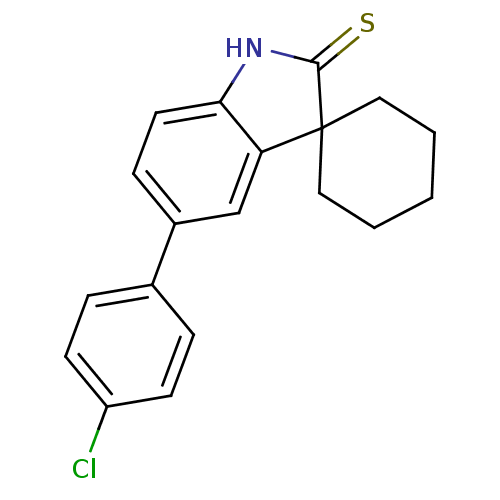 (CHEMBL25429) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404232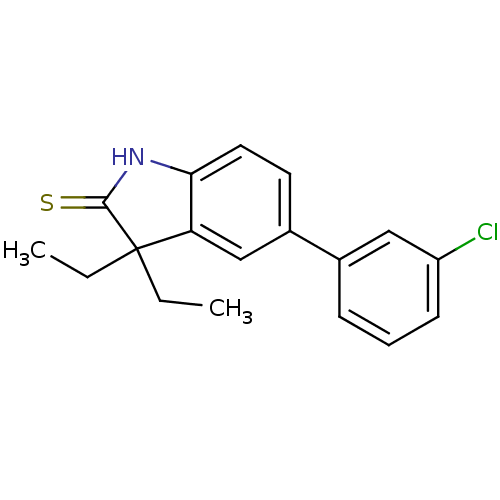 (CHEMBL25430) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404214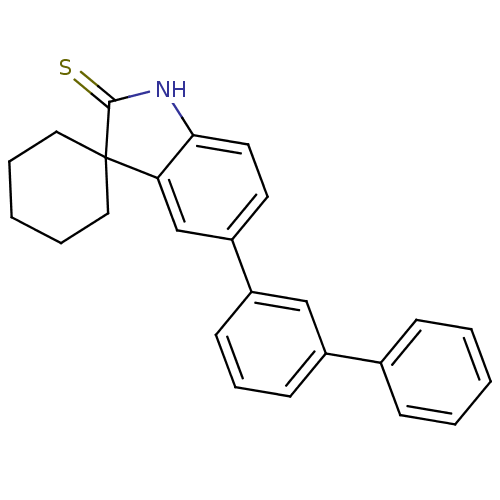 (CHEMBL412874) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404216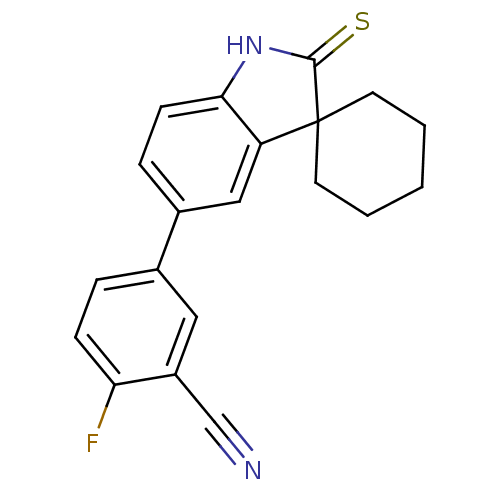 (CHEMBL282774) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404227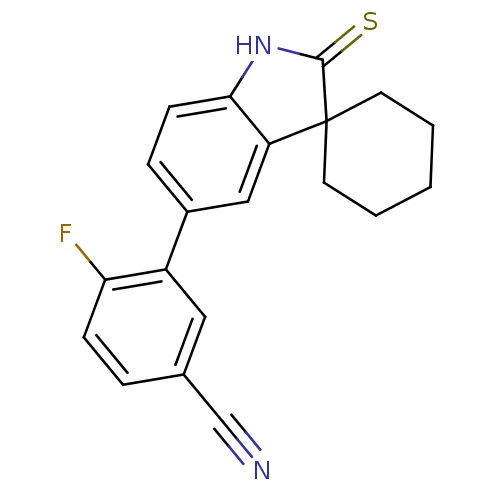 (CHEMBL28102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404231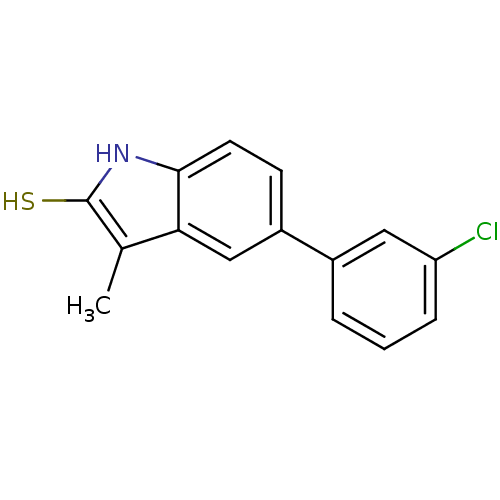 (CHEMBL28256) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404233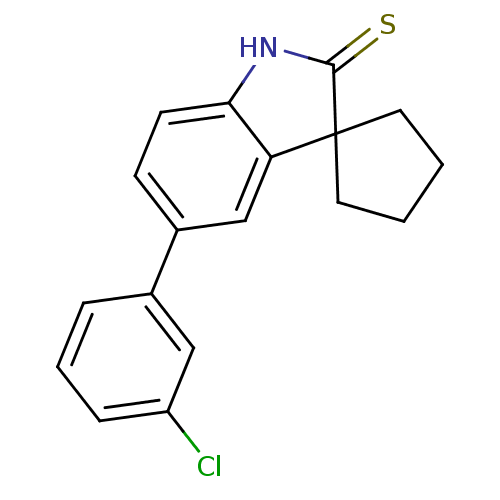 (CHEMBL283759) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404237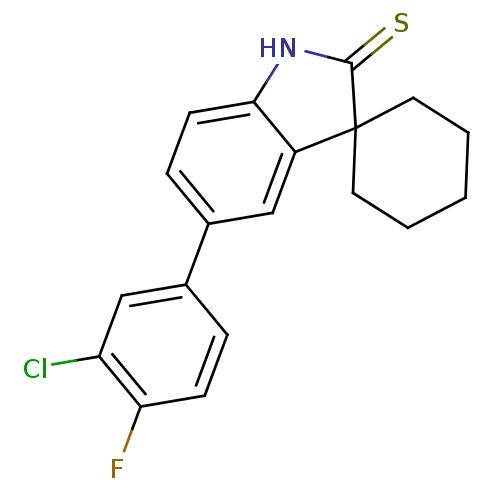 (CHEMBL26997) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404239 (CHEMBL28031) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50126158 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404213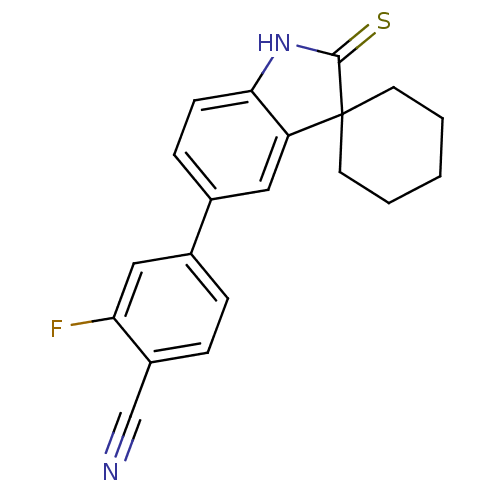 (CHEMBL26770) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404238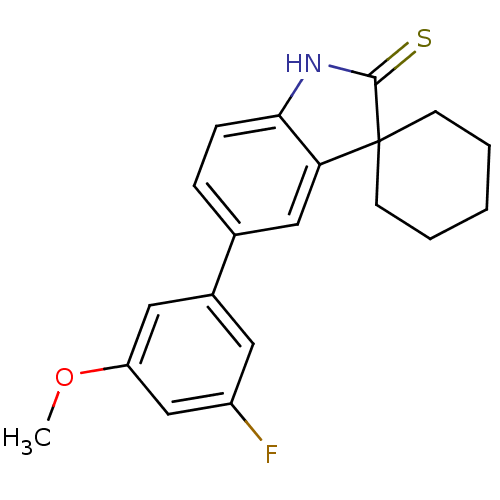 (CHEMBL286029) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concnetration against Androgen receptor in mouse fibroblast L929 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50126158 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration against glucocorticoid receptor in human lung A549 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404217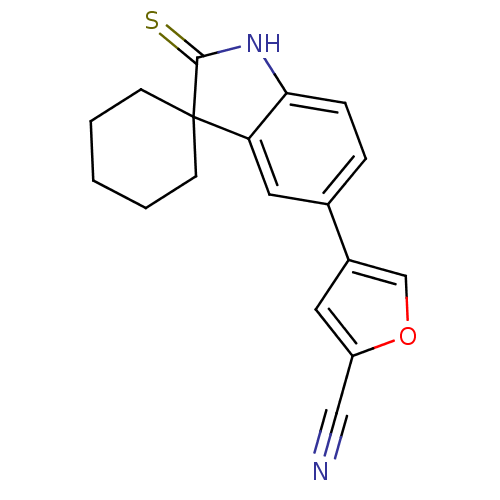 (CHEMBL280767) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375837 (CHEMBL270976) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404221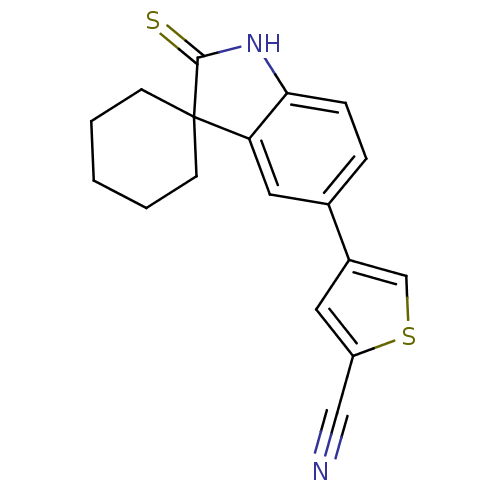 (CHEMBL285139) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404222 (CHEMBL417475) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404226 (CHEMBL287428) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404236 (CHEMBL28213) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404228 (CHEMBL28161) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404220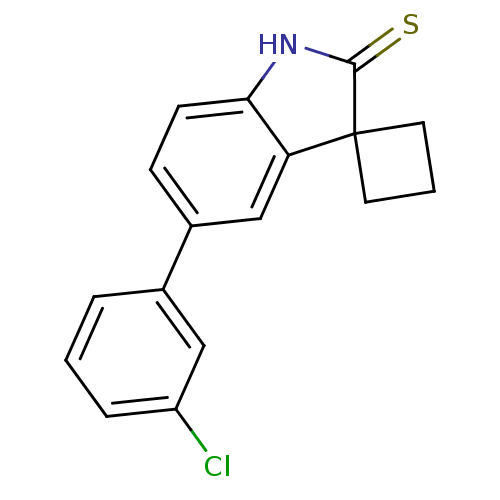 (CHEMBL25261) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404211 (CHEMBL25182) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity against glucocorticoid receptor in human lung A549 cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404218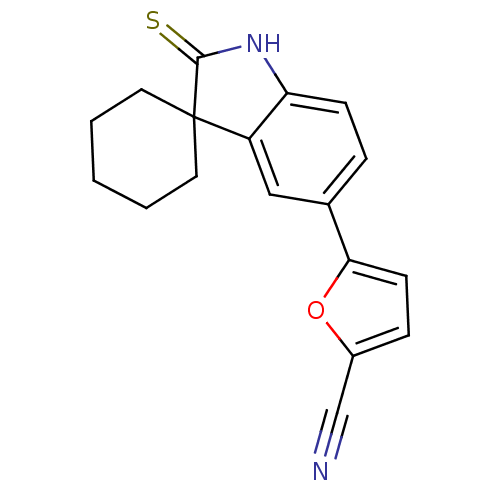 (CHEMBL27766) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||