

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828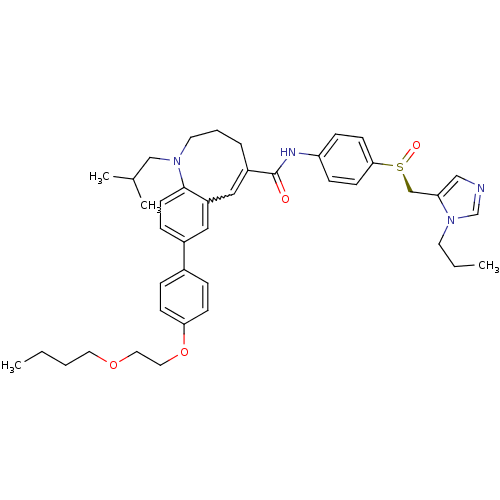 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396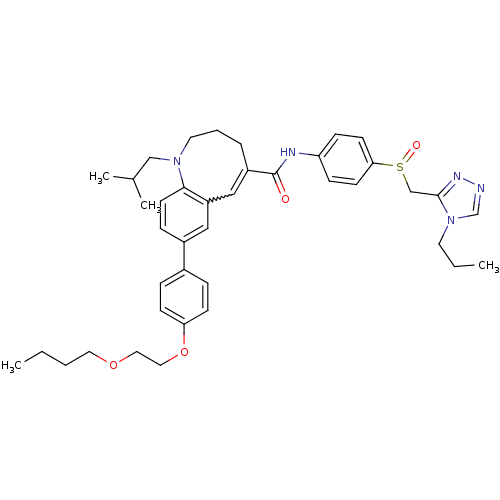 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399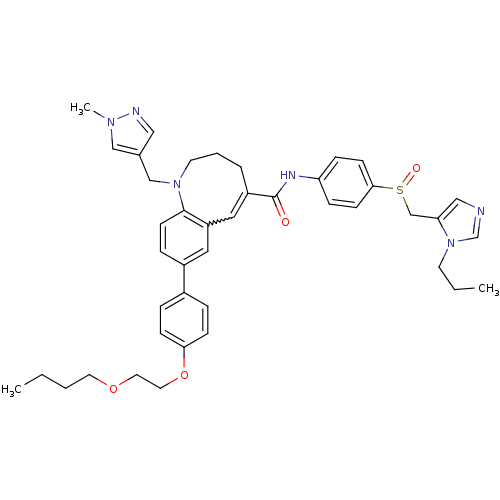 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398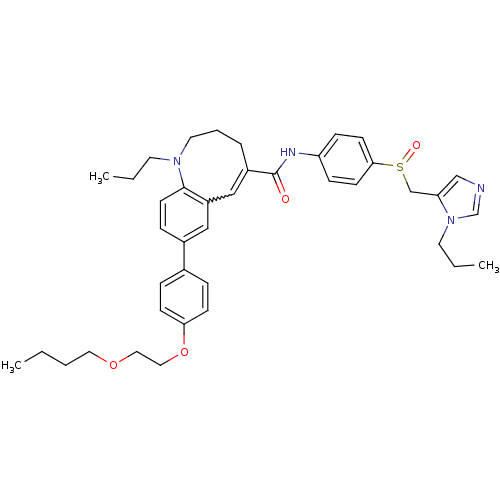 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400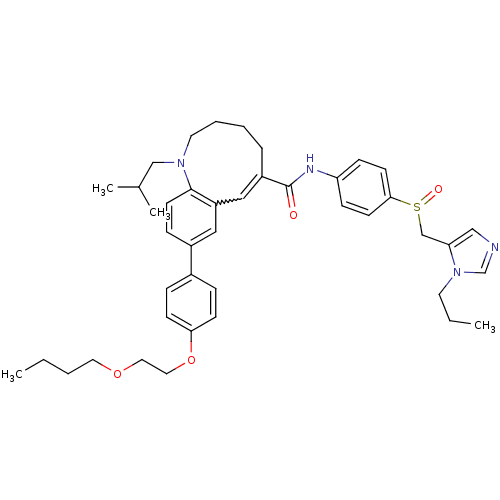 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402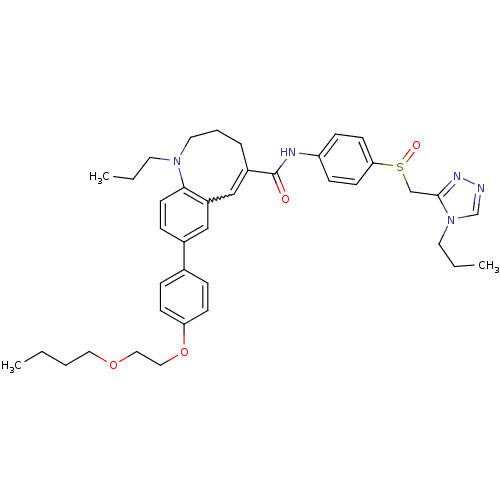 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403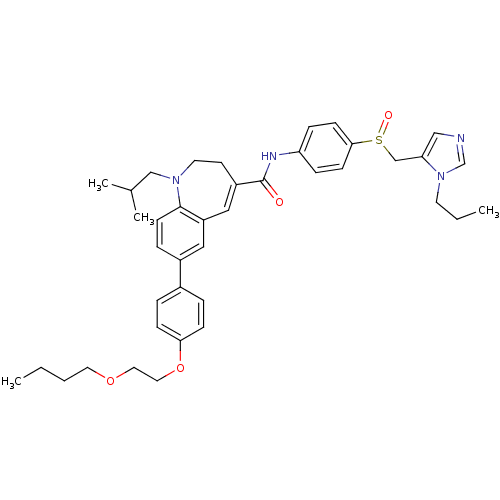 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184401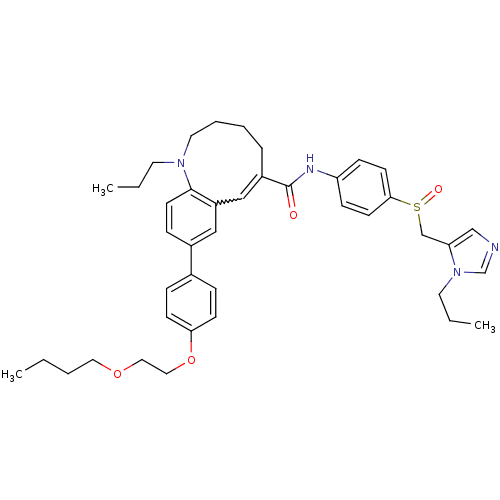 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCP-1 from CCR2b expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184404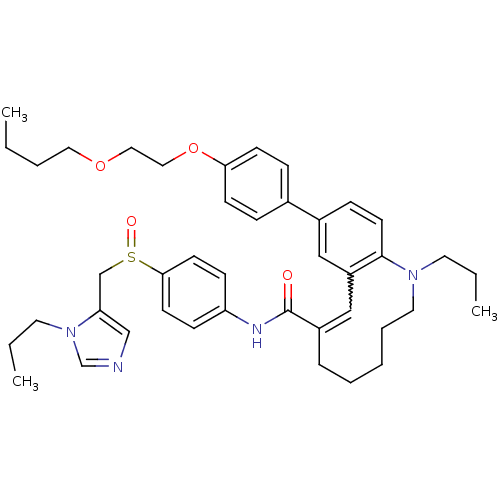 ((S)-(-)-10-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184405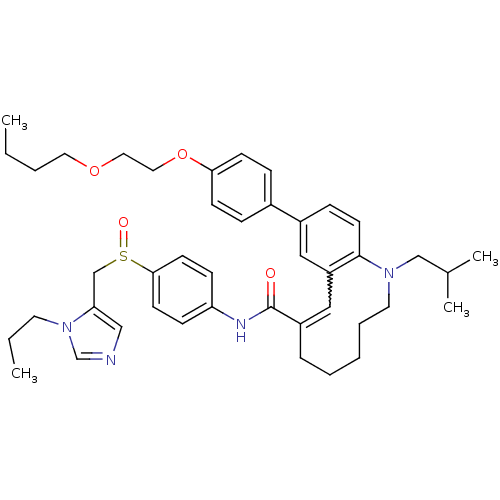 ((S)-(-)-10-{4-[2-( butoxy)ethoxy]phenyl}-1-isobuty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]TARC from CCR4 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]eotaxin from CCR3 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 7 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MIP-3beta from CCR7 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR1 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||