

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000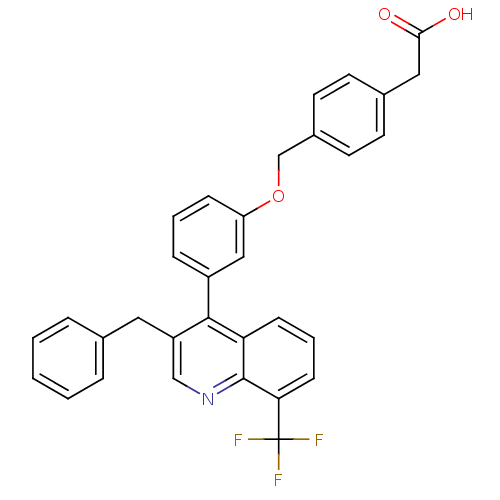 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35094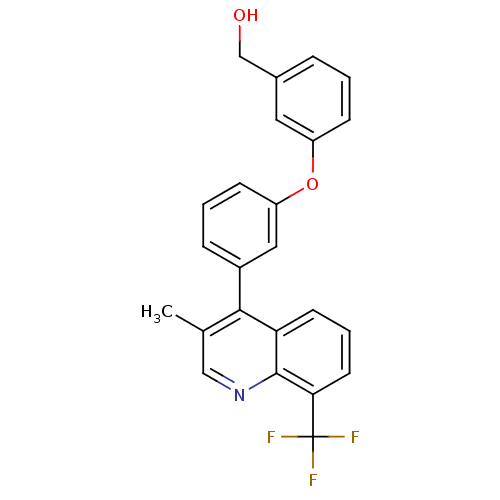 (biarylether alcohol quinoline, 5f) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | 233 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35099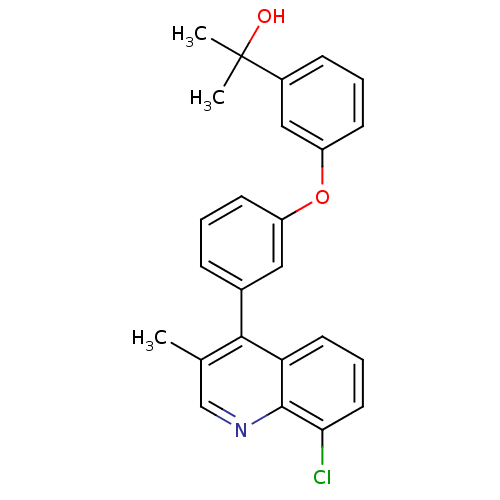 (biarylether alcohol quinoline, 5i) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | 108 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35089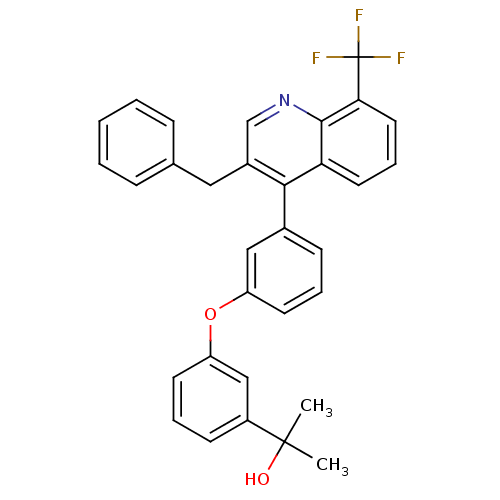 (biarylether alcohol quinoline, 5b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | 31 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35089 (biarylether alcohol quinoline, 5b) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35099 (biarylether alcohol quinoline, 5i) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35092 (biarylether alcohol quinoline, 5e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | 1.01E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 9 | n/a | 16 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35091 (biarylether alcohol quinoline, 5d) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 646 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35094 (biarylether alcohol quinoline, 5f) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35088 (biarylether alcohol quinoline, 5a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | 1.03E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35098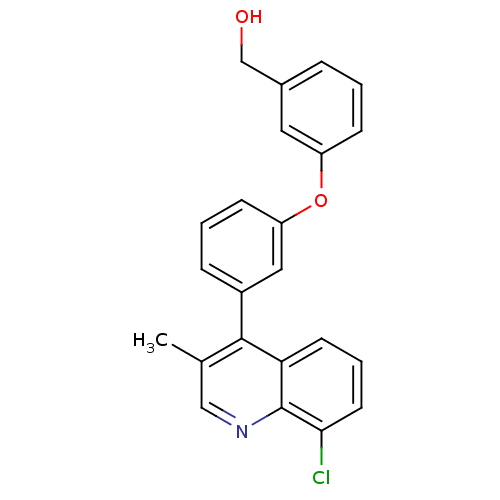 (biarylether alcohol quinoline, 5h) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 938 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35090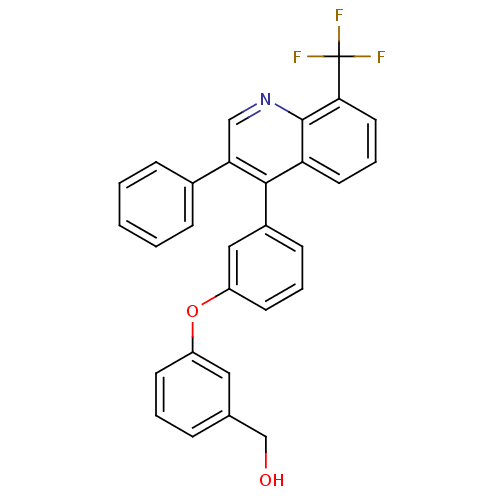 (biarylether alcohol quinoline, 5c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | 210 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35101 (biarylether alcohol quinoline, 9b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | 406 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35096 (biarylether alcohol quinoline, 5g) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | 1.37E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35092 (biarylether alcohol quinoline, 5e) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35090 (biarylether alcohol quinoline, 5c) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35087 (BMC171663 Compound 4a | Benzylquinoline, 8a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | 895 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35098 (biarylether alcohol quinoline, 5h) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35088 (biarylether alcohol quinoline, 5a) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35091 (biarylether alcohol quinoline, 5d) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35101 (biarylether alcohol quinoline, 9b) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35096 (biarylether alcohol quinoline, 5g) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35087 (BMC171663 Compound 4a | Benzylquinoline, 8a) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35097 (3-methyl quinoline, 8d | BMC171663 Compound 4h) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | 1.81E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35100 (biarylether methoxy quinoline, 9a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 287 | n/a | 612 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35093 (3-methyl quinoline, 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 435 | n/a | 3.40E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35100 (biarylether methoxy quinoline, 9a) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35095 (3-methyl quinoline, 8c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35093 (3-methyl quinoline, 8b) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35095 (3-methyl quinoline, 8c) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM35097 (3-methyl quinoline, 8d | BMC171663 Compound 4h) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRalpha coated flash plates. Each concentration of... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||