

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359284 (CHEMBL1928379) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359293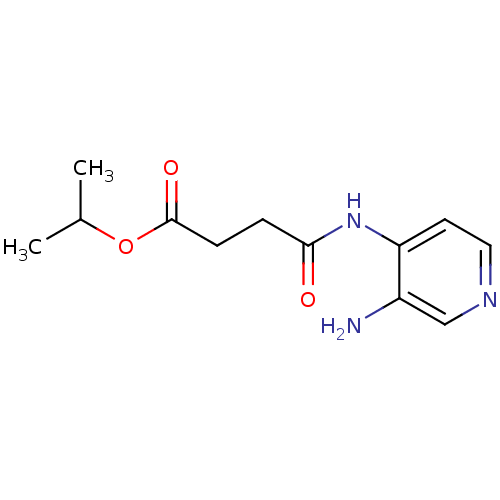 (CHEMBL1928397) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359287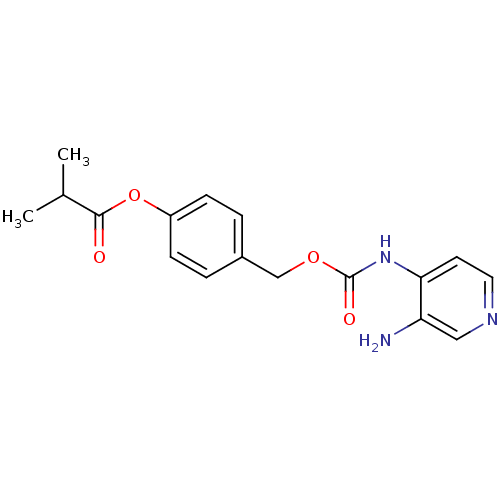 (CHEMBL1928382) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359285 (CHEMBL1928380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359289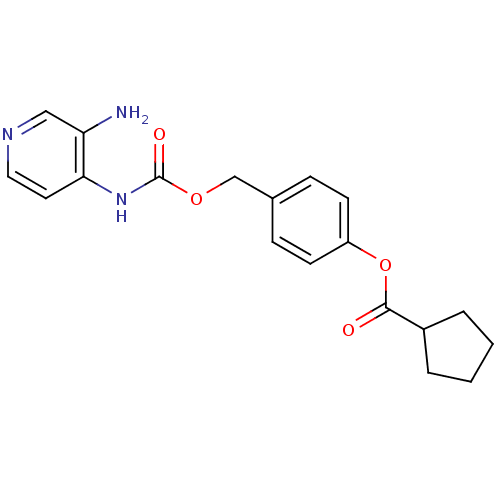 (CHEMBL1928384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359284 (CHEMBL1928379) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359286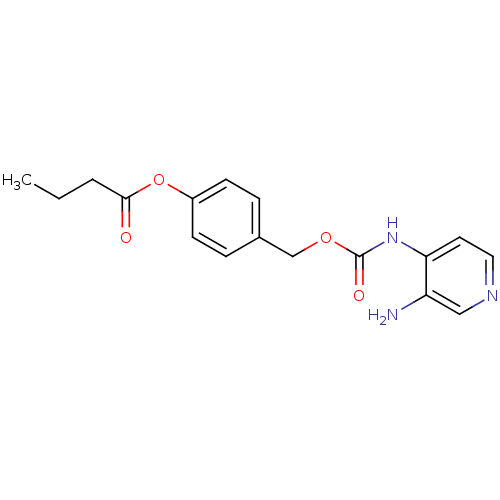 (CHEMBL1928381) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359286 (CHEMBL1928381) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359285 (CHEMBL1928380) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359294 (CHEMBL1928387) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359292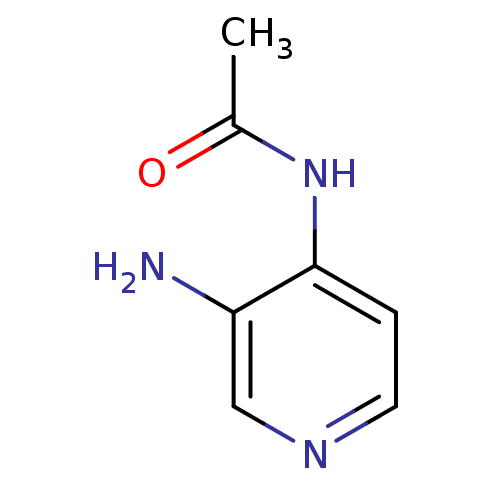 (CHEMBL1928393) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359289 (CHEMBL1928384) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359288 (CHEMBL1928383) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359287 (CHEMBL1928382) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359288 (CHEMBL1928383) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359293 (CHEMBL1928397) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359292 (CHEMBL1928393) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359291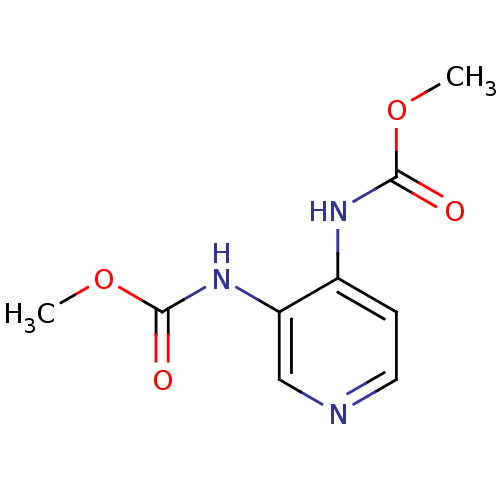 (CHEMBL1928391) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359290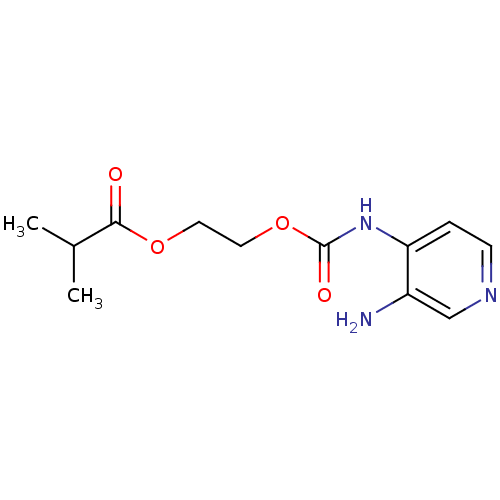 (CHEMBL1928389) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359290 (CHEMBL1928389) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359291 (CHEMBL1928391) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359285 (CHEMBL1928380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359287 (CHEMBL1928382) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359284 (CHEMBL1928379) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359289 (CHEMBL1928384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359288 (CHEMBL1928383) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359290 (CHEMBL1928389) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359294 (CHEMBL1928387) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359300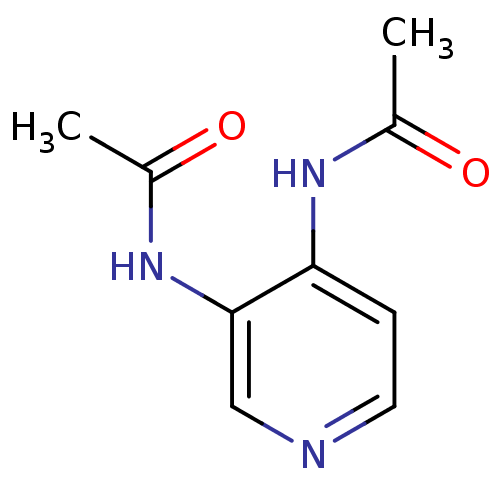 (CHEMBL1928394) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359291 (CHEMBL1928391) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359299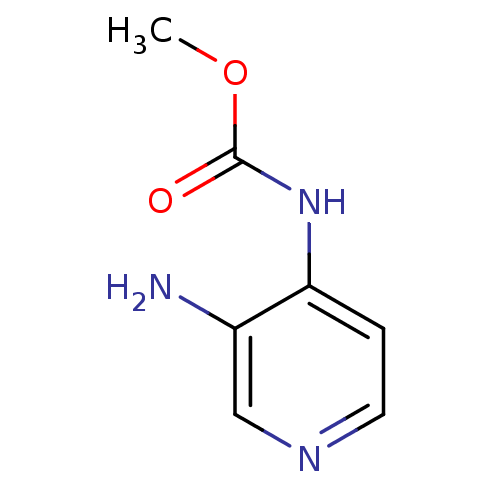 (CHEMBL1928392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359286 (CHEMBL1928381) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359284 (CHEMBL1928379) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359292 (CHEMBL1928393) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359296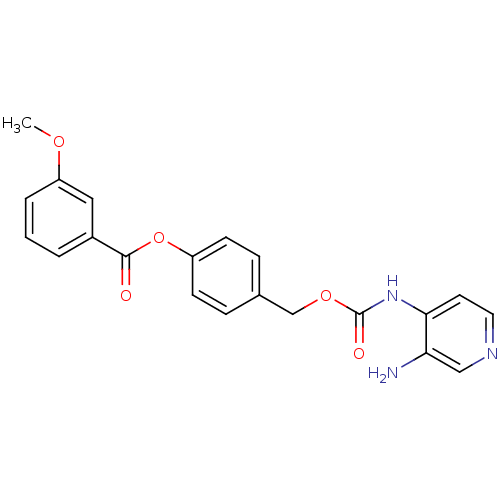 (CHEMBL1928386) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359290 (CHEMBL1928389) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359289 (CHEMBL1928384) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359288 (CHEMBL1928383) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359293 (CHEMBL1928397) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359293 (CHEMBL1928397) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359286 (CHEMBL1928381) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359287 (CHEMBL1928382) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359285 (CHEMBL1928380) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359294 (CHEMBL1928387) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359296 (CHEMBL1928386) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359295 (CHEMBL1928385) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359295 (CHEMBL1928385) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359300 (CHEMBL1928394) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359291 (CHEMBL1928391) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359299 (CHEMBL1928392) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359297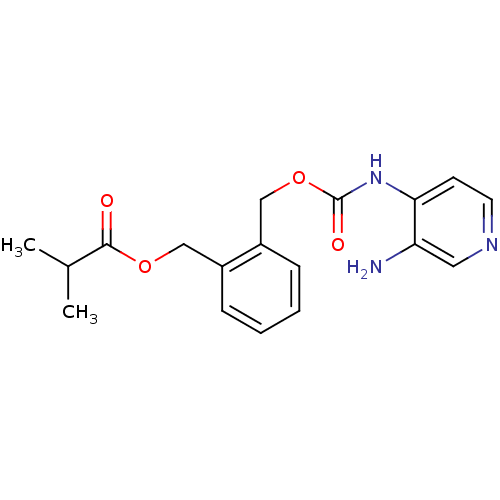 (CHEMBL1928388) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359302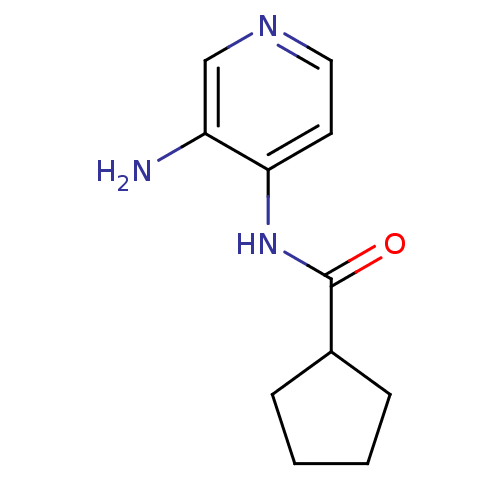 (CHEMBL1928396) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359301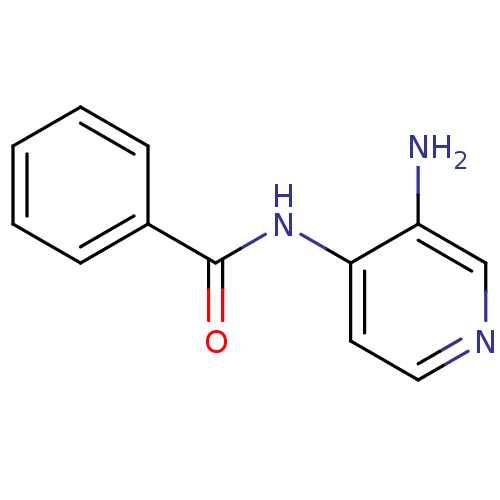 (CHEMBL1928395) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359298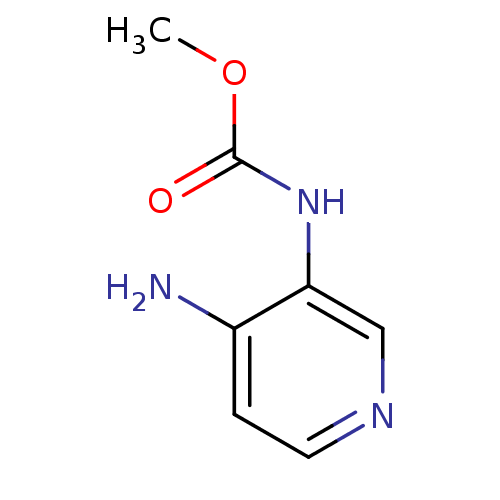 (CHEMBL1928390) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359297 (CHEMBL1928388) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359301 (CHEMBL1928395) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359302 (CHEMBL1928396) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359298 (CHEMBL1928390) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50359292 (CHEMBL1928393) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assay | Bioorg Med Chem 19: 6203-9 (2011) Article DOI: 10.1016/j.bmc.2011.09.019 BindingDB Entry DOI: 10.7270/Q25B02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||