

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM98678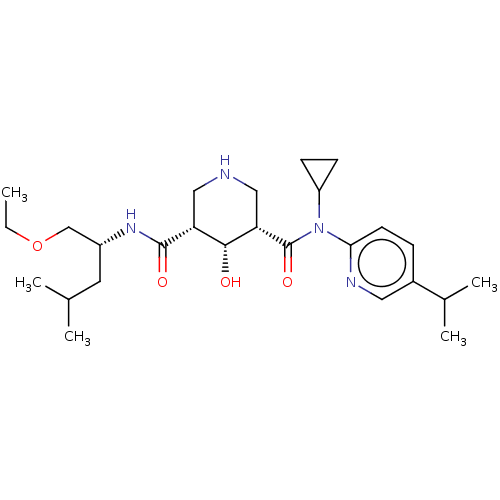 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684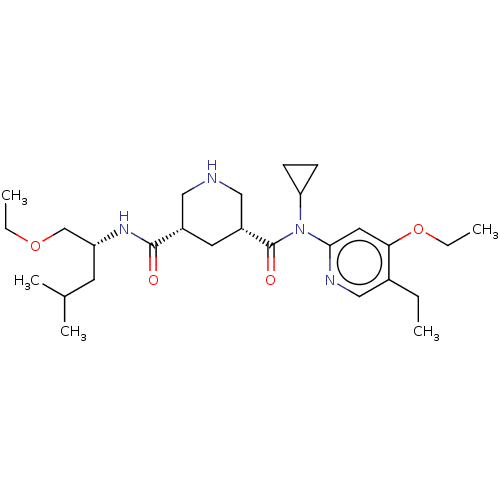 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054632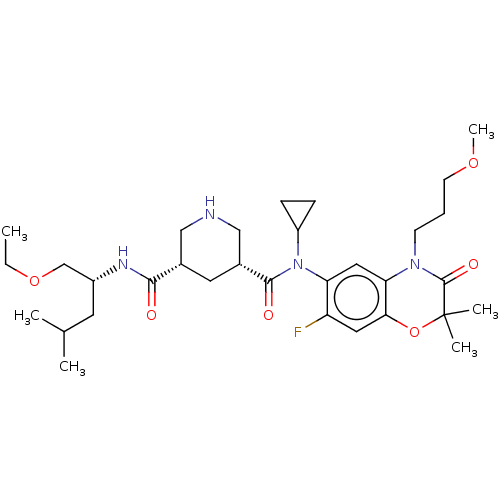 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540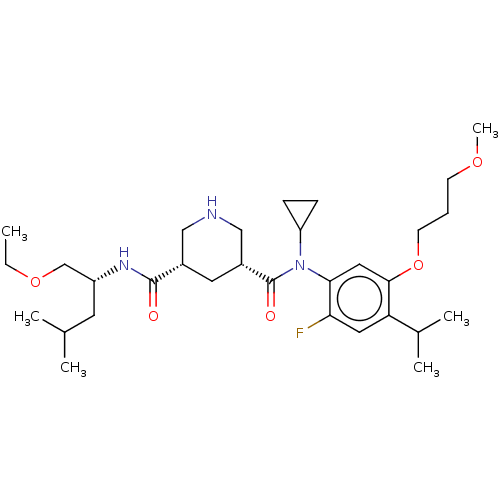 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054637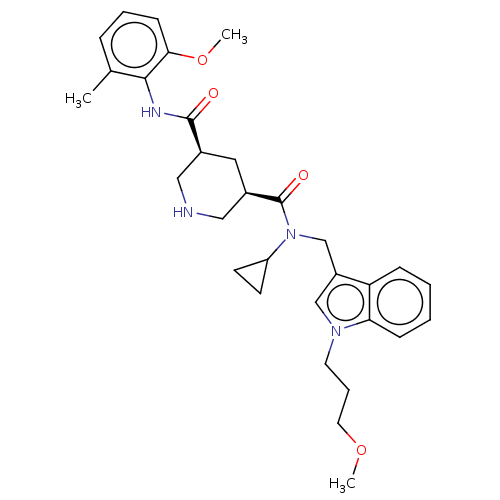 (CHEMBL3318938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054634 (CHEMBL3318942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054637 (CHEMBL3318938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054642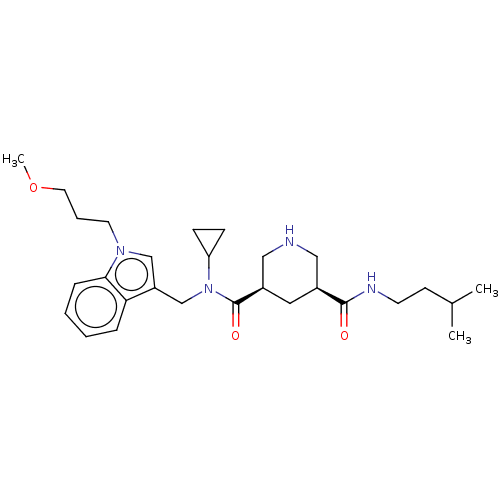 (CHEMBL3318935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054635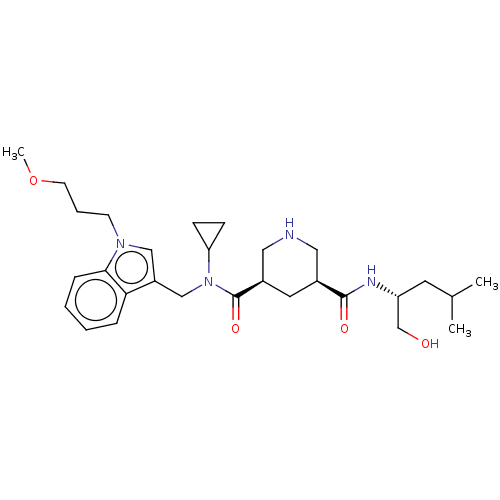 (CHEMBL3318936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054632 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054639 (CHEMBL3318929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054634 (CHEMBL3318942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054635 (CHEMBL3318936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054641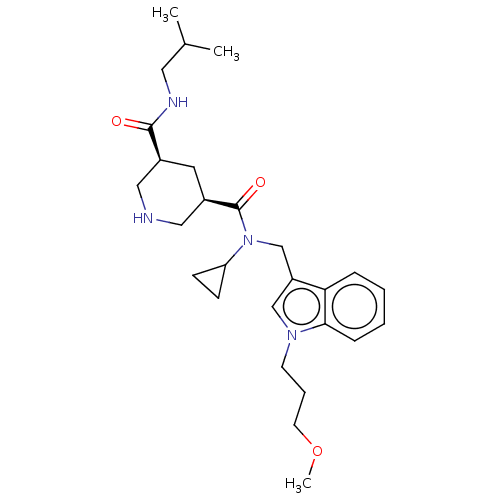 (CHEMBL3318934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054642 (CHEMBL3318935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054641 (CHEMBL3318934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054644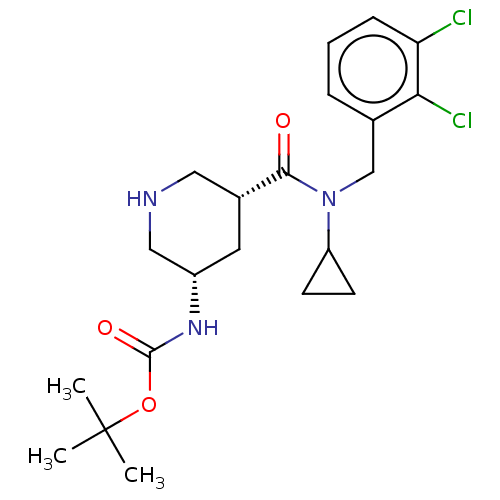 (CHEMBL3318932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054643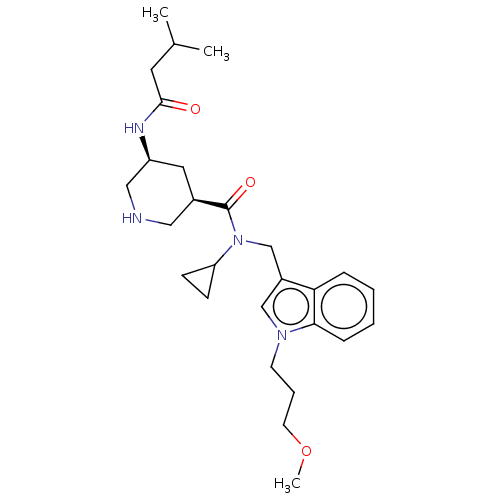 (CHEMBL3318933) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054640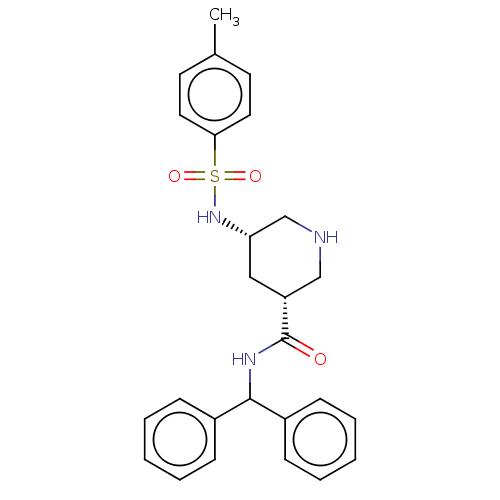 (CHEMBL3318930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054645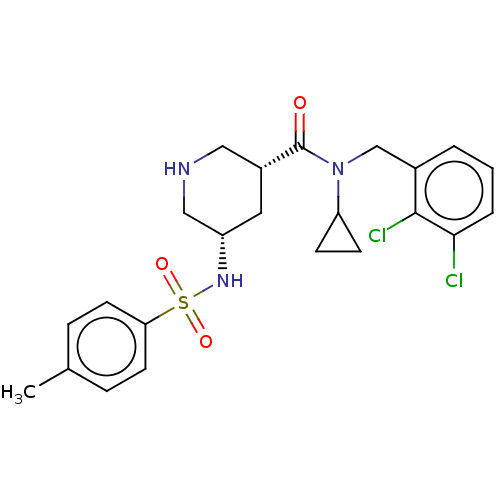 (CHEMBL3318931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50054634 (CHEMBL3318942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054639 (CHEMBL3318929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50054540 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054640 (CHEMBL3318930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Nav1.2 (unknown origin) by patch clamp assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant human procathepsin E expressed in Escherichia coli using fluorescence-quenched Ac-E-D(EDANS)-KPILFFRLG-K(Dabcyl)-E-Am... | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A-5 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant pepsinogen A expressed in Escherichia coli using fluorescence-quenched Dabcyl-E-R-Nle-F-L-S-F-P-EDANS substrate by fl... | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant procathepsin D expressed in SF9 cells using fluorescence-quenched Ac-E-D(EDANS)-KPILFFRLG-K(Dabcyl)-E-Amid substrate ... | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50054632 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE2 expressed in SF9 cells using fluorescence-quenched RE(EDANS)-EVNLDAEF-K(DABSYL)-R substrate by fluorimetric ass... | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Nav1.5 (unknown origin) by patch clamp assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant pepsinogen C expressed in Escherichia coli using fluorescence-quenched Dabcyl-E-R-Nle-F-L-S-F-P-EDANS substrate by fl... | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 expressed in SF9 cells using fluorescence-quenched RE(EDANS)-EVNLDAEF-K(DABSYL)-R substrate by fluorimetric ass... | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting method | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||