 Found 93 hits of Enzyme Inhibition Constant Data
Found 93 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245396
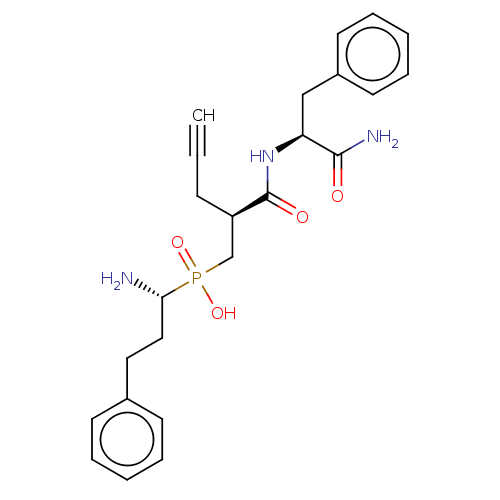
(CHEMBL4101200)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC#C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H30N3O4P/c1-2-9-20(17-32(30,31)22(25)15-14-18-10-5-3-6-11-18)24(29)27-21(23(26)28)16-19-12-7-4-8-13-19/h1,3-8,10-13,20-22H,9,14-17,25H2,(H2,26,28)(H,27,29)(H,30,31)/t20-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245391

(CHEMBL4095882)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O6P/c32-29(16-11-21-7-3-1-4-8-21)42(39,40)20-24(18-26-19-27(35-41-26)23-12-14-25(36)15-13-23)31(38)34-28(30(33)37)17-22-9-5-2-6-10-22/h1-10,12-15,19,24,28-29,36H,11,16-18,20,32H2,(H2,33,37)(H,34,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245390

(CHEMBL4077855)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O6P/c32-29(16-15-21-9-3-1-4-10-21)42(39,40)20-23(18-24-19-26(35-41-24)25-13-7-8-14-28(25)36)31(38)34-27(30(33)37)17-22-11-5-2-6-12-22/h1-14,19,23,27,29,36H,15-18,20,32H2,(H2,33,37)(H,34,38)(H,39,40)/t23-,27+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245400
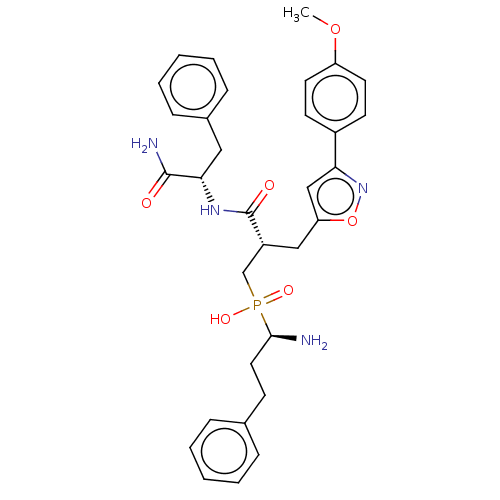
(CHEMBL4099107)Show SMILES COc1ccc(cc1)-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-26-15-13-24(14-16-26)28-20-27(42-36-28)19-25(21-43(39,40)30(33)17-12-22-8-4-2-5-9-22)32(38)35-29(31(34)37)18-23-10-6-3-7-11-23/h2-11,13-16,20,25,29-30H,12,17-19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t25-,29+,30-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245382
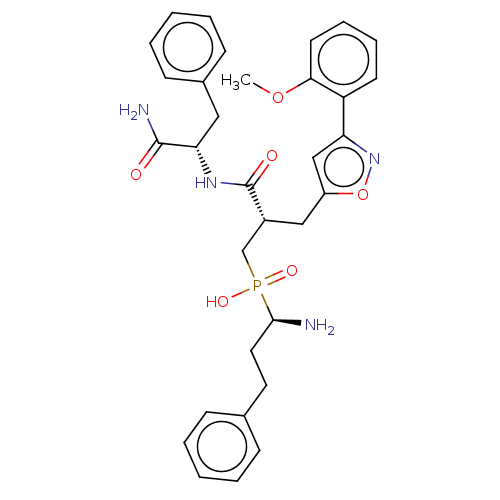
(CHEMBL4080145)Show SMILES COc1ccccc1-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-29-15-9-8-14-26(29)27-20-25(42-36-27)19-24(21-43(39,40)30(33)17-16-22-10-4-2-5-11-22)32(38)35-28(31(34)37)18-23-12-6-3-7-13-23/h2-15,20,24,28,30H,16-19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,30-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245388

(CHEMBL4081073)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1Cl)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-26-14-8-7-13-25(26)27-19-24(41-36-27)18-23(20-42(39,40)29(33)16-15-21-9-3-1-4-10-21)31(38)35-28(30(34)37)17-22-11-5-2-6-12-22/h1-14,19,23,28-29H,15-18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t23-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245349
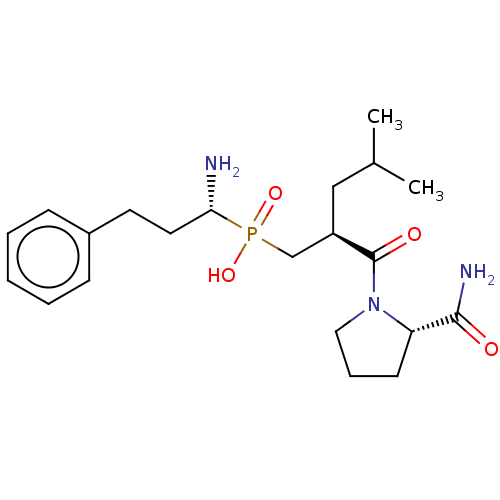
(CHEMBL4089555)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H34N3O4P/c1-15(2)13-17(21(26)24-12-6-9-18(24)20(23)25)14-29(27,28)19(22)11-10-16-7-4-3-5-8-16/h3-5,7-8,15,17-19H,6,9-14,22H2,1-2H3,(H2,23,25)(H,27,28)/t17-,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245428

(CHEMBL4069576)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(c1ccccc1)c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H42N3O4P/c1-23(2)20-29(31(34)36)35-32(37)27(22-40(38,39)30(33)19-18-24-12-6-3-7-13-24)21-28(25-14-8-4-9-15-25)26-16-10-5-11-17-26/h3-17,23,27-30H,18-22,33H2,1-2H3,(H2,34,36)(H,35,37)(H,38,39)/t27-,29+,30-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245399

(CHEMBL4069425)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O5P/c32-29(17-16-22-10-4-1-5-11-22)41(38,39)21-25(19-26-20-27(35-40-26)24-14-8-3-9-15-24)31(37)34-28(30(33)36)18-23-12-6-2-7-13-23/h1-15,20,25,28-29H,16-19,21,32H2,(H2,33,36)(H,34,37)(H,38,39)/t25-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245383

(CHEMBL4104383)Show SMILES COc1cccc(c1)-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-26-14-8-13-24(18-26)28-20-27(42-36-28)19-25(21-43(39,40)30(33)16-15-22-9-4-2-5-10-22)32(38)35-29(31(34)37)17-23-11-6-3-7-12-23/h2-14,18,20,25,29-30H,15-17,19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t25-,29+,30-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245446
(CHEMBL4094131)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H42N3O4P/c41-38(22-21-29-13-5-1-6-14-29)48(46,47)28-36(40(45)43-37(39(42)44)26-30-15-7-2-8-16-30)25-31-23-34(32-17-9-3-10-18-32)27-35(24-31)33-19-11-4-12-20-33/h1-20,23-24,27,36-38H,21-22,25-26,28,41H2,(H2,42,44)(H,43,45)(H,46,47)/t36-,37+,38-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245354

(CHEMBL4097268)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O5P/c1-17(2)14-20(16-34(32,33)23(26)13-10-18-6-4-3-5-7-18)25(31)28-22(24(27)30)15-19-8-11-21(29)12-9-19/h3-9,11-12,17,20,22-23,29H,10,13-16,26H2,1-2H3,(H2,27,30)(H,28,31)(H,32,33)/t20-,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50236826
(CHEMBL4065841)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H40N3O4P/c36-33(22-21-26-13-5-1-6-14-26)43(41,42)25-30(35(40)38-32(34(37)39)23-27-15-7-2-8-16-27)24-31(28-17-9-3-10-18-28)29-19-11-4-12-20-29/h1-20,30-33H,21-25,36H2,(H2,37,39)(H,38,40)(H,41,42)/t30-,32+,33-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245347
(CHEMBL4081682)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C22H38N3O4P/c1-15(2)12-18(22(27)25-19(21(24)26)13-16(3)4)14-30(28,29)20(23)11-10-17-8-6-5-7-9-17/h5-9,15-16,18-20H,10-14,23H2,1-4H3,(H2,24,26)(H,25,27)(H,28,29)/t18-,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245391

(CHEMBL4095882)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O6P/c32-29(16-11-21-7-3-1-4-8-21)42(39,40)20-24(18-26-19-27(35-41-26)23-12-14-25(36)15-13-23)31(38)34-28(30(33)37)17-22-9-5-2-6-10-22/h1-10,12-15,19,24,28-29,36H,11,16-18,20,32H2,(H2,33,37)(H,34,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245401
(CHEMBL4103640)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-25-14-12-23(13-15-25)27-19-26(41-36-27)18-24(20-42(39,40)29(33)16-11-21-7-3-1-4-8-21)31(38)35-28(30(34)37)17-22-9-5-2-6-10-22/h1-10,12-15,19,24,28-29H,11,16-18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245396

(CHEMBL4101200)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC#C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H30N3O4P/c1-2-9-20(17-32(30,31)22(25)15-14-18-10-5-3-6-11-18)24(29)27-21(23(26)28)16-19-12-7-4-8-13-19/h1,3-8,10-13,20-22H,9,14-17,25H2,(H2,26,28)(H,27,29)(H,30,31)/t20-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245389

(CHEMBL4085707)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-25-13-7-12-23(17-25)27-19-26(41-36-27)18-24(20-42(39,40)29(33)15-14-21-8-3-1-4-9-21)31(38)35-28(30(34)37)16-22-10-5-2-6-11-22/h1-13,17,19,24,28-29H,14-16,18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245348

(CHEMBL4099693)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O4P/c1-18(2)15-21(17-33(31,32)23(26)14-13-19-9-5-3-6-10-19)25(30)28-22(24(27)29)16-20-11-7-4-8-12-20/h3-12,18,21-23H,13-17,26H2,1-2H3,(H2,27,29)(H,28,30)(H,31,32)/t21-,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245396

(CHEMBL4101200)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC#C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H30N3O4P/c1-2-9-20(17-32(30,31)22(25)15-14-18-10-5-3-6-11-18)24(29)27-21(23(26)28)16-19-12-7-4-8-13-19/h1,3-8,10-13,20-22H,9,14-17,25H2,(H2,26,28)(H,27,29)(H,30,31)/t20-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245402

(CHEMBL4074464)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,THB:21:22:19.20.25:17,21:20:17:23.22.24,15:16:19:23.22.21,24:22:19:25.16.17,24:16:19:23.22.21| Show InChI InChI=1S/C32H44N3O4P/c33-29(12-11-22-7-3-1-4-8-22)40(38,39)21-27(20-32-17-24-13-25(18-32)15-26(14-24)19-32)31(37)35-28(30(34)36)16-23-9-5-2-6-10-23/h1-10,24-29H,11-21,33H2,(H2,34,36)(H,35,37)(H,38,39)/t24?,25?,26?,27-,28+,29-,32?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245400

(CHEMBL4099107)Show SMILES COc1ccc(cc1)-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-26-15-13-24(14-16-26)28-20-27(42-36-28)19-25(21-43(39,40)30(33)17-12-22-8-4-2-5-9-22)32(38)35-29(31(34)37)18-23-10-6-3-7-11-23/h2-11,13-16,20,25,29-30H,12,17-19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t25-,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245354

(CHEMBL4097268)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O5P/c1-17(2)14-20(16-34(32,33)23(26)13-10-18-6-4-3-5-7-18)25(31)28-22(24(27)30)15-19-8-11-21(29)12-9-19/h3-9,11-12,17,20,22-23,29H,10,13-16,26H2,1-2H3,(H2,27,30)(H,28,31)(H,32,33)/t20-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245391

(CHEMBL4095882)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O6P/c32-29(16-11-21-7-3-1-4-8-21)42(39,40)20-24(18-26-19-27(35-41-26)23-12-14-25(36)15-13-23)31(38)34-28(30(33)37)17-22-9-5-2-6-10-22/h1-10,12-15,19,24,28-29,36H,11,16-18,20,32H2,(H2,33,37)(H,34,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245390

(CHEMBL4077855)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O6P/c32-29(16-15-21-9-3-1-4-10-21)42(39,40)20-23(18-24-19-26(35-41-24)25-13-7-8-14-28(25)36)31(38)34-27(30(33)37)17-22-11-5-2-6-12-22/h1-14,19,23,27,29,36H,15-18,20,32H2,(H2,33,37)(H,34,38)(H,39,40)/t23-,27+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245401

(CHEMBL4103640)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-25-14-12-23(13-15-25)27-19-26(41-36-27)18-24(20-42(39,40)29(33)16-11-21-7-3-1-4-8-21)31(38)35-28(30(34)37)17-22-9-5-2-6-10-22/h1-10,12-15,19,24,28-29H,11,16-18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245390

(CHEMBL4077855)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O6P/c32-29(16-15-21-9-3-1-4-10-21)42(39,40)20-23(18-24-19-26(35-41-24)25-13-7-8-14-28(25)36)31(38)34-27(30(33)37)17-22-11-5-2-6-12-22/h1-14,19,23,27,29,36H,15-18,20,32H2,(H2,33,37)(H,34,38)(H,39,40)/t23-,27+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245400

(CHEMBL4099107)Show SMILES COc1ccc(cc1)-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-26-15-13-24(14-16-26)28-20-27(42-36-28)19-25(21-43(39,40)30(33)17-12-22-8-4-2-5-9-22)32(38)35-29(31(34)37)18-23-10-6-3-7-11-23/h2-11,13-16,20,25,29-30H,12,17-19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t25-,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245460
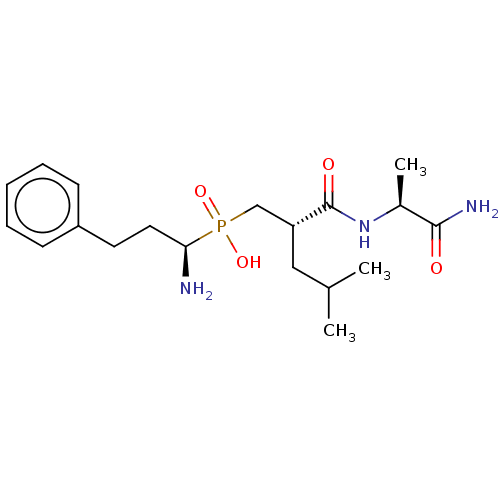
(CHEMBL4091951)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C19H32N3O4P/c1-13(2)11-16(19(24)22-14(3)18(21)23)12-27(25,26)17(20)10-9-15-7-5-4-6-8-15/h4-8,13-14,16-17H,9-12,20H2,1-3H3,(H2,21,23)(H,22,24)(H,25,26)/t14-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245383

(CHEMBL4104383)Show SMILES COc1cccc(c1)-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-26-14-8-13-24(18-26)28-20-27(42-36-28)19-25(21-43(39,40)30(33)16-15-22-9-4-2-5-10-22)32(38)35-29(31(34)37)17-23-11-6-3-7-12-23/h2-14,18,20,25,29-30H,15-17,19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t25-,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245460

(CHEMBL4091951)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C19H32N3O4P/c1-13(2)11-16(19(24)22-14(3)18(21)23)12-27(25,26)17(20)10-9-15-7-5-4-6-8-15/h4-8,13-14,16-17H,9-12,20H2,1-3H3,(H2,21,23)(H,22,24)(H,25,26)/t14-,16+,17+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245388

(CHEMBL4081073)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1Cl)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-26-14-8-7-13-25(26)27-19-24(41-36-27)18-23(20-42(39,40)29(33)16-15-21-9-3-1-4-10-21)31(38)35-28(30(34)37)17-22-11-5-2-6-12-22/h1-14,19,23,28-29H,15-18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t23-,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245446

(CHEMBL4094131)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H42N3O4P/c41-38(22-21-29-13-5-1-6-14-29)48(46,47)28-36(40(45)43-37(39(42)44)26-30-15-7-2-8-16-30)25-31-23-34(32-17-9-3-10-18-32)27-35(24-31)33-19-11-4-12-20-33/h1-20,23-24,27,36-38H,21-22,25-26,28,41H2,(H2,42,44)(H,43,45)(H,46,47)/t36-,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245348

(CHEMBL4099693)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O4P/c1-18(2)15-21(17-33(31,32)23(26)14-13-19-9-5-3-6-10-19)25(30)28-22(24(27)29)16-20-11-7-4-8-12-20/h3-12,18,21-23H,13-17,26H2,1-2H3,(H2,27,29)(H,28,30)(H,31,32)/t21-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245347

(CHEMBL4081682)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C22H38N3O4P/c1-15(2)12-18(22(27)25-19(21(24)26)13-16(3)4)14-30(28,29)20(23)11-10-17-8-6-5-7-9-17/h5-9,15-16,18-20H,10-14,23H2,1-4H3,(H2,24,26)(H,25,27)(H,28,29)/t18-,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245388

(CHEMBL4081073)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1Cl)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-26-14-8-7-13-25(26)27-19-24(41-36-27)18-23(20-42(39,40)29(33)16-15-21-9-3-1-4-10-21)31(38)35-28(30(34)37)17-22-11-5-2-6-12-22/h1-14,19,23,28-29H,15-18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t23-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245399

(CHEMBL4069425)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O5P/c32-29(17-16-22-10-4-1-5-11-22)41(38,39)21-25(19-26-20-27(35-40-26)24-14-8-3-9-15-24)31(37)34-28(30(33)36)18-23-12-6-2-7-13-23/h1-15,20,25,28-29H,16-19,21,32H2,(H2,33,36)(H,34,37)(H,38,39)/t25-,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245356

(CHEMBL4066723)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O |r| Show InChI InChI=1S/C22H34N5O4P/c1-15(2)10-17(22(29)27-19(21(24)28)11-18-12-25-14-26-18)13-32(30,31)20(23)9-8-16-6-4-3-5-7-16/h3-7,12,14-15,17,19-20H,8-11,13,23H2,1-2H3,(H2,24,28)(H,25,26)(H,27,29)(H,30,31)/t17-,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245355
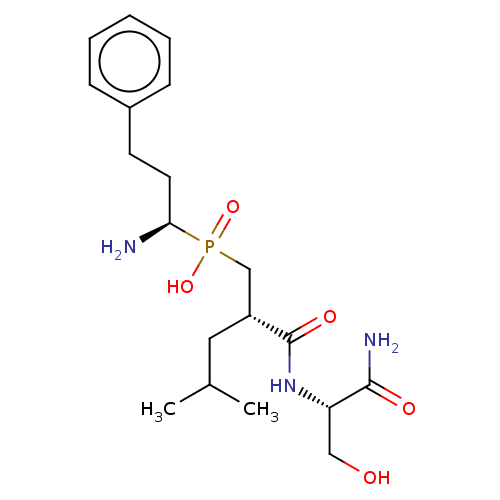
(CHEMBL4070078)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C19H32N3O5P/c1-13(2)10-15(19(25)22-16(11-23)18(21)24)12-28(26,27)17(20)9-8-14-6-4-3-5-7-14/h3-7,13,15-17,23H,8-12,20H2,1-2H3,(H2,21,24)(H,22,25)(H,26,27)/t15-,16+,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245382

(CHEMBL4080145)Show SMILES COc1ccccc1-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-29-15-9-8-14-26(29)27-20-25(42-36-27)19-24(21-43(39,40)30(33)17-16-22-10-4-2-5-11-22)32(38)35-28(31(34)37)18-23-12-6-3-7-13-23/h2-15,20,24,28,30H,16-19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245389

(CHEMBL4085707)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-25-13-7-12-23(17-25)27-19-26(41-36-27)18-24(20-42(39,40)29(33)15-14-21-8-3-1-4-9-21)31(38)35-28(30(34)37)16-22-10-5-2-6-11-22/h1-13,17,19,24,28-29H,14-16,18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245349

(CHEMBL4089555)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H34N3O4P/c1-15(2)13-17(21(26)24-12-6-9-18(24)20(23)25)14-29(27,28)19(22)11-10-16-7-4-3-5-8-16/h3-5,7-8,15,17-19H,6,9-14,22H2,1-2H3,(H2,23,25)(H,27,28)/t17-,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245348

(CHEMBL4099693)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O4P/c1-18(2)15-21(17-33(31,32)23(26)14-13-19-9-5-3-6-10-19)25(30)28-22(24(27)29)16-20-11-7-4-8-12-20/h3-12,18,21-23H,13-17,26H2,1-2H3,(H2,27,29)(H,28,30)(H,31,32)/t21-,22+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245382

(CHEMBL4080145)Show SMILES COc1ccccc1-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-29-15-9-8-14-26(29)27-20-25(42-36-27)19-24(21-43(39,40)30(33)17-16-22-10-4-2-5-11-22)32(38)35-28(31(34)37)18-23-12-6-3-7-13-23/h2-15,20,24,28,30H,16-19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245380

(CHEMBL4077711)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O4P/c1-18(2)15-21(17-33(31,32)23(26)14-13-19-9-5-3-6-10-19)25(30)28-22(24(27)29)16-20-11-7-4-8-12-20/h3-12,18,21-23H,13-17,26H2,1-2H3,(H2,27,29)(H,28,30)(H,31,32)/t21-,22-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245399

(CHEMBL4069425)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O5P/c32-29(17-16-22-10-4-1-5-11-22)41(38,39)21-25(19-26-20-27(35-40-26)24-14-8-3-9-15-24)31(37)34-28(30(33)36)18-23-12-6-2-7-13-23/h1-15,20,25,28-29H,16-19,21,32H2,(H2,33,36)(H,34,37)(H,38,39)/t25-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245380

(CHEMBL4077711)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O4P/c1-18(2)15-21(17-33(31,32)23(26)14-13-19-9-5-3-6-10-19)25(30)28-22(24(27)29)16-20-11-7-4-8-12-20/h3-12,18,21-23H,13-17,26H2,1-2H3,(H2,27,29)(H,28,30)(H,31,32)/t21-,22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245383

(CHEMBL4104383)Show SMILES COc1cccc(c1)-c1cc(C[C@H](CP(O)(=O)[C@@H](N)CCc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)on1 |r| Show InChI InChI=1S/C32H37N4O6P/c1-41-26-14-8-13-24(18-26)28-20-27(42-36-28)19-25(21-43(39,40)30(33)16-15-22-9-4-2-5-10-22)32(38)35-29(31(34)37)17-23-11-6-3-7-12-23/h2-14,18,20,25,29-30H,15-17,19,21,33H2,1H3,(H2,34,37)(H,35,38)(H,39,40)/t25-,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245427

(CHEMBL4096799)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](CC(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H40N3O4P/c36-33(22-21-26-13-5-1-6-14-26)43(41,42)25-30(35(40)38-32(34(37)39)23-27-15-7-2-8-16-27)24-31(28-17-9-3-10-18-28)29-19-11-4-12-20-29/h1-20,30-33H,21-25,36H2,(H2,37,39)(H,38,40)(H,41,42)/t30-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245389

(CHEMBL4085707)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-25-13-7-12-23(17-25)27-19-26(41-36-27)18-24(20-42(39,40)29(33)15-14-21-8-3-1-4-9-21)31(38)35-28(30(34)37)16-22-10-5-2-6-11-22/h1-13,17,19,24,28-29H,14-16,18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245366

(CHEMBL4095735)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C21H34N3O6P/c1-14(2)12-16(21(28)24-17(20(23)27)9-11-19(25)26)13-31(29,30)18(22)10-8-15-6-4-3-5-7-15/h3-7,14,16-18H,8-13,22H2,1-2H3,(H2,23,27)(H,24,28)(H,25,26)(H,29,30)/t16-,17+,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245428

(CHEMBL4069576)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(c1ccccc1)c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H42N3O4P/c1-23(2)20-29(31(34)36)35-32(37)27(22-40(38,39)30(33)19-18-24-12-6-3-7-13-24)21-28(25-14-8-4-9-15-25)26-16-10-5-11-17-26/h3-17,23,27-30H,18-22,33H2,1-2H3,(H2,34,36)(H,35,37)(H,38,39)/t27-,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245398
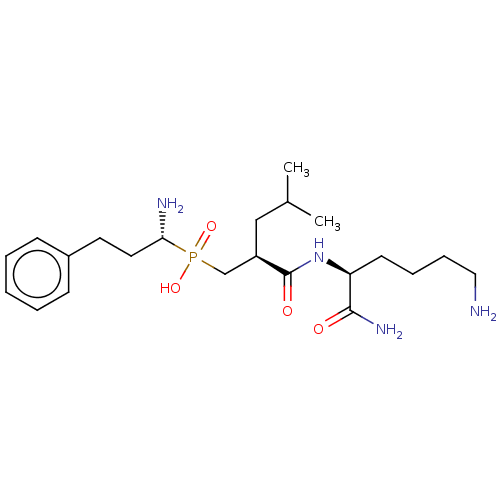
(CHEMBL4088020)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C22H39N4O4P/c1-16(2)14-18(22(28)26-19(21(25)27)10-6-7-13-23)15-31(29,30)20(24)12-11-17-8-4-3-5-9-17/h3-5,8-9,16,18-20H,6-7,10-15,23-24H2,1-2H3,(H2,25,27)(H,26,28)(H,29,30)/t18-,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245429

(CHEMBL4073490)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H42N3O4P/c41-38(22-21-29-13-5-1-6-14-29)48(46,47)28-36(40(45)43-37(39(42)44)26-30-15-7-2-8-16-30)25-31-23-34(32-17-9-3-10-18-32)27-35(24-31)33-19-11-4-12-20-33/h1-20,23-24,27,36-38H,21-22,25-26,28,41H2,(H2,42,44)(H,43,45)(H,46,47)/t36-,37-,38+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245430
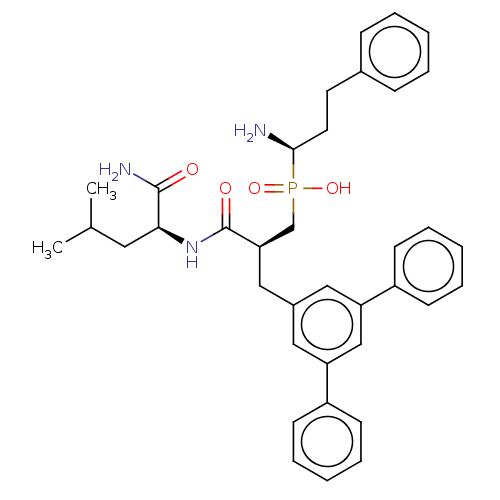
(CHEMBL4086352)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H44N3O4P/c1-26(2)20-34(36(39)41)40-37(42)33(25-45(43,44)35(38)19-18-27-12-6-3-7-13-27)23-28-21-31(29-14-8-4-9-15-29)24-32(22-28)30-16-10-5-11-17-30/h3-17,21-22,24,26,33-35H,18-20,23,25,38H2,1-2H3,(H2,39,41)(H,40,42)(H,43,44)/t33-,34+,35-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 317 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245354

(CHEMBL4097268)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O5P/c1-17(2)14-20(16-34(32,33)23(26)13-10-18-6-4-3-5-7-18)25(31)28-22(24(27)30)15-19-8-11-21(29)12-9-19/h3-9,11-12,17,20,22-23,29H,10,13-16,26H2,1-2H3,(H2,27,30)(H,28,31)(H,32,33)/t20-,22+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245401

(CHEMBL4103640)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(no1)-c1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H34ClN4O5P/c32-25-14-12-23(13-15-25)27-19-26(41-36-27)18-24(20-42(39,40)29(33)16-11-21-7-3-1-4-8-21)31(38)35-28(30(34)37)17-22-9-5-2-6-10-22/h1-10,12-15,19,24,28-29H,11,16-18,20,33H2,(H2,34,37)(H,35,38)(H,39,40)/t24-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245356

(CHEMBL4066723)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O |r| Show InChI InChI=1S/C22H34N5O4P/c1-15(2)10-17(22(29)27-19(21(24)28)11-18-12-25-14-26-18)13-32(30,31)20(23)9-8-16-6-4-3-5-7-16/h3-7,12,14-15,17,19-20H,8-11,13,23H2,1-2H3,(H2,24,28)(H,25,26)(H,27,29)(H,30,31)/t17-,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245402

(CHEMBL4074464)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,THB:21:22:19.20.25:17,21:20:17:23.22.24,15:16:19:23.22.21,24:22:19:25.16.17,24:16:19:23.22.21| Show InChI InChI=1S/C32H44N3O4P/c33-29(12-11-22-7-3-1-4-8-22)40(38,39)21-27(20-32-17-24-13-25(18-32)15-26(14-24)19-32)31(37)35-28(30(34)36)16-23-9-5-2-6-10-23/h1-10,24-29H,11-21,33H2,(H2,34,36)(H,35,37)(H,38,39)/t24?,25?,26?,27-,28+,29-,32?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245381
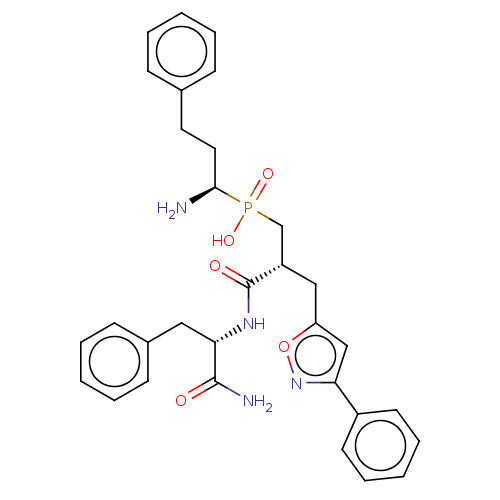
(CHEMBL4096656)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](Cc1cc(no1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O5P/c32-29(17-16-22-10-4-1-5-11-22)41(38,39)21-25(19-26-20-27(35-40-26)24-14-8-3-9-15-24)31(37)34-28(30(33)36)18-23-12-6-2-7-13-23/h1-15,20,25,28-29H,16-19,21,32H2,(H2,33,36)(H,34,37)(H,38,39)/t25-,28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 434 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245397

(CHEMBL4093454)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](CC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,TLB:15:16:19:23.22.21,THB:17:16:19.18.23:21,17:18:21:25.16.24,24:16:19:23.22.21,24:22:19:25.16.17,15:16:19.18.23:21| Show InChI InChI=1S/C32H44N3O4P/c33-29(12-11-22-7-3-1-4-8-22)40(38,39)21-27(20-32-17-24-13-25(18-32)15-26(14-24)19-32)31(37)35-28(30(34)36)16-23-9-5-2-6-10-23/h1-10,24-29H,11-21,33H2,(H2,34,36)(H,35,37)(H,38,39)/t24?,25?,26?,27-,28-,29+,32?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 561 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245437

(CHEMBL4064891)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1cccc(Cl)c1)-c1cccc(Cl)c1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H42Cl2N3O4P/c1-24(2)16-34(36(41)43)42-37(44)31(23-47(45,46)35(40)15-14-25-8-4-3-5-9-25)19-26-17-29(27-10-6-12-32(38)21-27)20-30(18-26)28-11-7-13-33(39)22-28/h3-13,17-18,20-22,24,31,34-35H,14-16,19,23,40H2,1-2H3,(H2,41,43)(H,42,44)(H,45,46)/t31-,34+,35-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 577 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245347

(CHEMBL4081682)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C22H38N3O4P/c1-15(2)12-18(22(27)25-19(21(24)26)13-16(3)4)14-30(28,29)20(23)11-10-17-8-6-5-7-9-17/h5-9,15-16,18-20H,10-14,23H2,1-4H3,(H2,24,26)(H,25,27)(H,28,29)/t18-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 682 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245402

(CHEMBL4074464)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,THB:21:22:19.20.25:17,21:20:17:23.22.24,15:16:19:23.22.21,24:22:19:25.16.17,24:16:19:23.22.21| Show InChI InChI=1S/C32H44N3O4P/c33-29(12-11-22-7-3-1-4-8-22)40(38,39)21-27(20-32-17-24-13-25(18-32)15-26(14-24)19-32)31(37)35-28(30(34)36)16-23-9-5-2-6-10-23/h1-10,24-29H,11-21,33H2,(H2,34,36)(H,35,37)(H,38,39)/t24?,25?,26?,27-,28+,29-,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 726 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50236826

(CHEMBL4065841)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H40N3O4P/c36-33(22-21-26-13-5-1-6-14-26)43(41,42)25-30(35(40)38-32(34(37)39)23-27-15-7-2-8-16-27)24-31(28-17-9-3-10-18-28)29-19-11-4-12-20-29/h1-20,30-33H,21-25,36H2,(H2,37,39)(H,38,40)(H,41,42)/t30-,32+,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245381

(CHEMBL4096656)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](Cc1cc(no1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O5P/c32-29(17-16-22-10-4-1-5-11-22)41(38,39)21-25(19-26-20-27(35-40-26)24-14-8-3-9-15-24)31(37)34-28(30(33)36)18-23-12-6-2-7-13-23/h1-15,20,25,28-29H,16-19,21,32H2,(H2,33,36)(H,34,37)(H,38,39)/t25-,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 878 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245349

(CHEMBL4089555)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H34N3O4P/c1-15(2)13-17(21(26)24-12-6-9-18(24)20(23)25)14-29(27,28)19(22)11-10-16-7-4-3-5-8-16/h3-5,7-8,15,17-19H,6,9-14,22H2,1-2H3,(H2,23,25)(H,27,28)/t17-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 949 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245355

(CHEMBL4070078)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C19H32N3O5P/c1-13(2)10-15(19(25)22-16(11-23)18(21)24)12-28(26,27)17(20)9-8-14-6-4-3-5-7-14/h3-7,13,15-17,23H,8-12,20H2,1-2H3,(H2,21,24)(H,22,25)(H,26,27)/t15-,16+,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245398

(CHEMBL4088020)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C22H39N4O4P/c1-16(2)14-18(22(28)26-19(21(25)27)10-6-7-13-23)15-31(29,30)20(24)12-11-17-8-4-3-5-9-17/h3-5,8-9,16,18-20H,6-7,10-15,23-24H2,1-2H3,(H2,25,27)(H,26,28)(H,29,30)/t18-,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245427

(CHEMBL4096799)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](CC(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H40N3O4P/c36-33(22-21-26-13-5-1-6-14-26)43(41,42)25-30(35(40)38-32(34(37)39)23-27-15-7-2-8-16-27)24-31(28-17-9-3-10-18-28)29-19-11-4-12-20-29/h1-20,30-33H,21-25,36H2,(H2,37,39)(H,38,40)(H,41,42)/t30-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245356

(CHEMBL4066723)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O |r| Show InChI InChI=1S/C22H34N5O4P/c1-15(2)10-17(22(29)27-19(21(24)28)11-18-12-25-14-26-18)13-32(30,31)20(23)9-8-16-6-4-3-5-7-16/h3-7,12,14-15,17,19-20H,8-11,13,23H2,1-2H3,(H2,24,28)(H,25,26)(H,27,29)(H,30,31)/t17-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245381

(CHEMBL4096656)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](Cc1cc(no1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C31H35N4O5P/c32-29(17-16-22-10-4-1-5-11-22)41(38,39)21-25(19-26-20-27(35-40-26)24-14-8-3-9-15-24)31(37)34-28(30(33)36)18-23-12-6-2-7-13-23/h1-15,20,25,28-29H,16-19,21,32H2,(H2,33,36)(H,34,37)(H,38,39)/t25-,28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245397

(CHEMBL4093454)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](CC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,TLB:15:16:19:23.22.21,THB:17:16:19.18.23:21,17:18:21:25.16.24,24:16:19:23.22.21,24:22:19:25.16.17,15:16:19.18.23:21| Show InChI InChI=1S/C32H44N3O4P/c33-29(12-11-22-7-3-1-4-8-22)40(38,39)21-27(20-32-17-24-13-25(18-32)15-26(14-24)19-32)31(37)35-28(30(34)36)16-23-9-5-2-6-10-23/h1-10,24-29H,11-21,33H2,(H2,34,36)(H,35,37)(H,38,39)/t24?,25?,26?,27-,28-,29+,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245460

(CHEMBL4091951)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C19H32N3O4P/c1-13(2)11-16(19(24)22-14(3)18(21)23)12-27(25,26)17(20)10-9-15-7-5-4-6-8-15/h4-8,13-14,16-17H,9-12,20H2,1-3H3,(H2,21,23)(H,22,24)(H,25,26)/t14-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245430

(CHEMBL4086352)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H44N3O4P/c1-26(2)20-34(36(39)41)40-37(42)33(25-45(43,44)35(38)19-18-27-12-6-3-7-13-27)23-28-21-31(29-14-8-4-9-15-29)24-32(22-28)30-16-10-5-11-17-30/h3-17,21-22,24,26,33-35H,18-20,23,25,38H2,1-2H3,(H2,39,41)(H,40,42)(H,43,44)/t33-,34+,35-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245380

(CHEMBL4077711)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C25H36N3O4P/c1-18(2)15-21(17-33(31,32)23(26)14-13-19-9-5-3-6-10-19)25(30)28-22(24(27)29)16-20-11-7-4-8-12-20/h3-12,18,21-23H,13-17,26H2,1-2H3,(H2,27,29)(H,28,30)(H,31,32)/t21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245366

(CHEMBL4095735)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C21H34N3O6P/c1-14(2)12-16(21(28)24-17(20(23)27)9-11-19(25)26)13-31(29,30)18(22)10-8-15-6-4-3-5-7-15/h3-7,14,16-18H,8-13,22H2,1-2H3,(H2,23,27)(H,24,28)(H,25,26)(H,29,30)/t16-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245398

(CHEMBL4088020)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C22H39N4O4P/c1-16(2)14-18(22(28)26-19(21(25)27)10-6-7-13-23)15-31(29,30)20(24)12-11-17-8-4-3-5-9-17/h3-5,8-9,16,18-20H,6-7,10-15,23-24H2,1-2H3,(H2,25,27)(H,26,28)(H,29,30)/t18-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50236826

(CHEMBL4065841)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](CC(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H40N3O4P/c36-33(22-21-26-13-5-1-6-14-26)43(41,42)25-30(35(40)38-32(34(37)39)23-27-15-7-2-8-16-27)24-31(28-17-9-3-10-18-28)29-19-11-4-12-20-29/h1-20,30-33H,21-25,36H2,(H2,37,39)(H,38,40)(H,41,42)/t30-,32+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50245366

(CHEMBL4095735)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C21H34N3O6P/c1-14(2)12-16(21(28)24-17(20(23)27)9-11-19(25)26)13-31(29,30)18(22)10-8-15-6-4-3-5-7-15/h3-7,14,16-18H,8-13,22H2,1-2H3,(H2,23,27)(H,24,28)(H,25,26)(H,29,30)/t16-,17+,18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245429

(CHEMBL4073490)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H42N3O4P/c41-38(22-21-29-13-5-1-6-14-29)48(46,47)28-36(40(45)43-37(39(42)44)26-30-15-7-2-8-16-30)25-31-23-34(32-17-9-3-10-18-32)27-35(24-31)33-19-11-4-12-20-33/h1-20,23-24,27,36-38H,21-22,25-26,28,41H2,(H2,42,44)(H,43,45)(H,46,47)/t36-,37-,38+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245355

(CHEMBL4070078)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C19H32N3O5P/c1-13(2)10-15(19(25)22-16(11-23)18(21)24)12-28(26,27)17(20)9-8-14-6-4-3-5-7-14/h3-7,13,15-17,23H,8-12,20H2,1-2H3,(H2,21,24)(H,22,25)(H,26,27)/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50245437

(CHEMBL4064891)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1cccc(Cl)c1)-c1cccc(Cl)c1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H42Cl2N3O4P/c1-24(2)16-34(36(41)43)42-37(44)31(23-47(45,46)35(40)15-14-25-8-4-3-5-9-25)19-26-17-29(27-10-6-12-32(38)21-27)20-30(18-26)28-11-7-13-33(39)22-28/h3-13,17-18,20-22,24,31,34-35H,14-16,19,23,40H2,1-2H3,(H2,41,43)(H,42,44)(H,45,46)/t31-,34+,35-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245430

(CHEMBL4086352)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H44N3O4P/c1-26(2)20-34(36(39)41)40-37(42)33(25-45(43,44)35(38)19-18-27-12-6-3-7-13-27)23-28-21-31(29-14-8-4-9-15-29)24-32(22-28)30-16-10-5-11-17-30/h3-17,21-22,24,26,33-35H,18-20,23,25,38H2,1-2H3,(H2,39,41)(H,40,42)(H,43,44)/t33-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245437

(CHEMBL4064891)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1cccc(Cl)c1)-c1cccc(Cl)c1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H42Cl2N3O4P/c1-24(2)16-34(36(41)43)42-37(44)31(23-47(45,46)35(40)15-14-25-8-4-3-5-9-25)19-26-17-29(27-10-6-12-32(38)21-27)20-30(18-26)28-11-7-13-33(39)22-28/h3-13,17-18,20-22,24,31,34-35H,14-16,19,23,40H2,1-2H3,(H2,41,43)(H,42,44)(H,45,46)/t31-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245397

(CHEMBL4093454)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](CC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,TLB:15:16:19:23.22.21,THB:17:16:19.18.23:21,17:18:21:25.16.24,24:16:19:23.22.21,24:22:19:25.16.17,15:16:19.18.23:21| Show InChI InChI=1S/C32H44N3O4P/c33-29(12-11-22-7-3-1-4-8-22)40(38,39)21-27(20-32-17-24-13-25(18-32)15-26(14-24)19-32)31(37)35-28(30(34)36)16-23-9-5-2-6-10-23/h1-10,24-29H,11-21,33H2,(H2,34,36)(H,35,37)(H,38,39)/t24?,25?,26?,27-,28-,29+,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245428

(CHEMBL4069576)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(c1ccccc1)c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H42N3O4P/c1-23(2)20-29(31(34)36)35-32(37)27(22-40(38,39)30(33)19-18-24-12-6-3-7-13-24)21-28(25-14-8-4-9-15-25)26-16-10-5-11-17-26/h3-17,23,27-30H,18-22,33H2,1-2H3,(H2,34,36)(H,35,37)(H,38,39)/t27-,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245446

(CHEMBL4094131)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H42N3O4P/c41-38(22-21-29-13-5-1-6-14-29)48(46,47)28-36(40(45)43-37(39(42)44)26-30-15-7-2-8-16-30)25-31-23-34(32-17-9-3-10-18-32)27-35(24-31)33-19-11-4-12-20-33/h1-20,23-24,27,36-38H,21-22,25-26,28,41H2,(H2,42,44)(H,43,45)(H,46,47)/t36-,37+,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245429

(CHEMBL4073490)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H42N3O4P/c41-38(22-21-29-13-5-1-6-14-29)48(46,47)28-36(40(45)43-37(39(42)44)26-30-15-7-2-8-16-30)25-31-23-34(32-17-9-3-10-18-32)27-35(24-31)33-19-11-4-12-20-33/h1-20,23-24,27,36-38H,21-22,25-26,28,41H2,(H2,42,44)(H,43,45)(H,46,47)/t36-,37-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50245427

(CHEMBL4096799)Show SMILES N[C@@H](CCc1ccccc1)P(O)(=O)C[C@H](CC(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H40N3O4P/c36-33(22-21-26-13-5-1-6-14-26)43(41,42)25-30(35(40)38-32(34(37)39)23-27-15-7-2-8-16-27)24-31(28-17-9-3-10-18-28)29-19-11-4-12-20-29/h1-20,30-33H,21-25,36H2,(H2,37,39)(H,38,40)(H,41,42)/t30-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assay |
J Med Chem 59: 9107-9123 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01031
BindingDB Entry DOI: 10.7270/Q2PK0JJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data