

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294419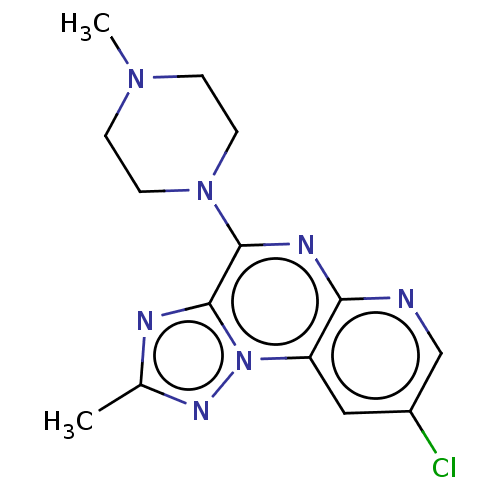 (US9586959, Compound 98) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294378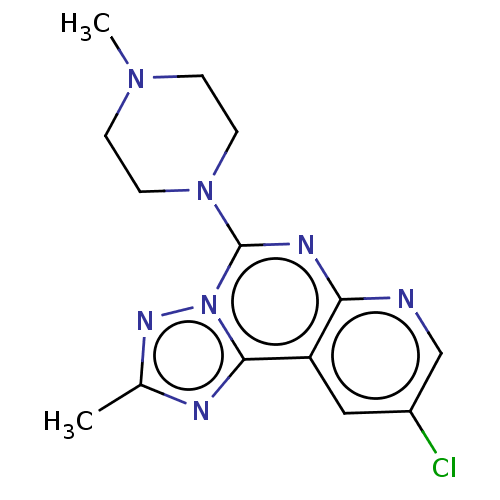 (US9586959, Compound 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294427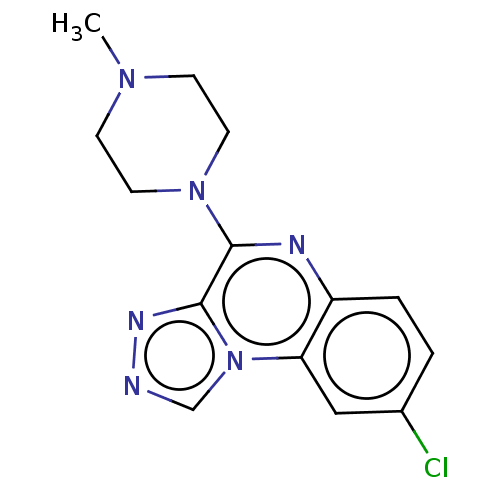 (US9586959, Compound disclosed in WO 2010030785, Ex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294418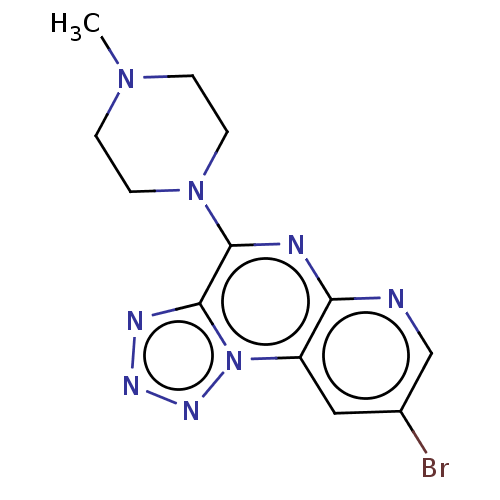 (US9586959, Compound 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294373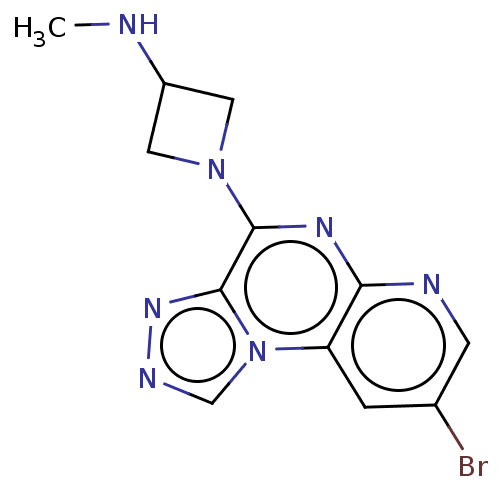 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294386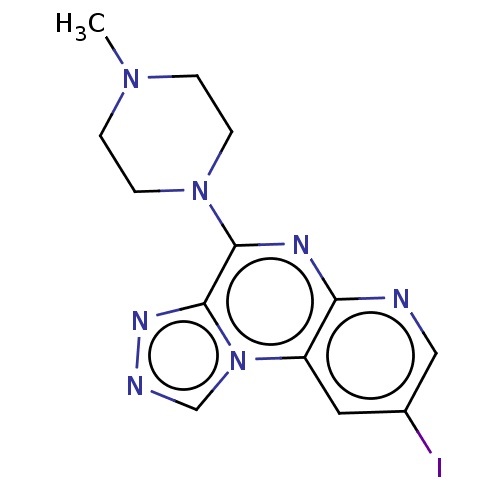 (US9586959, Compound 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407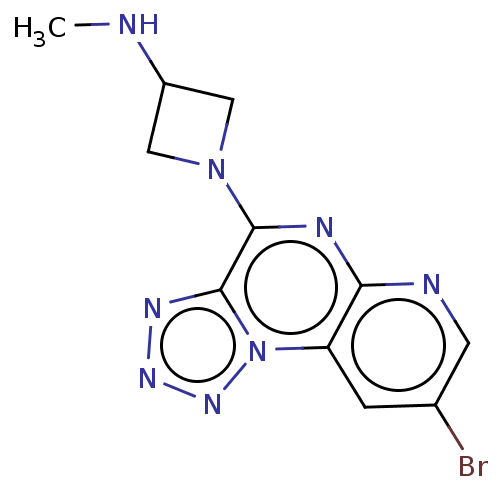 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294361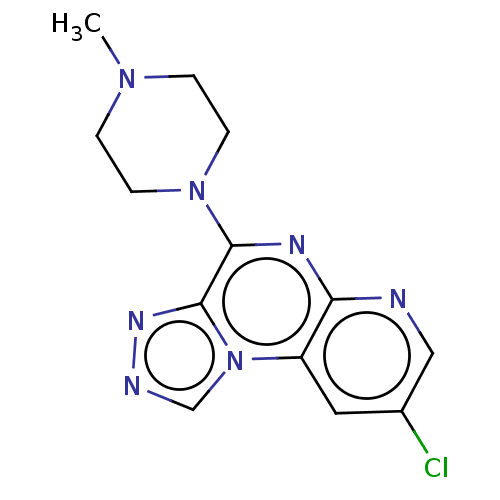 (US9586959, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294414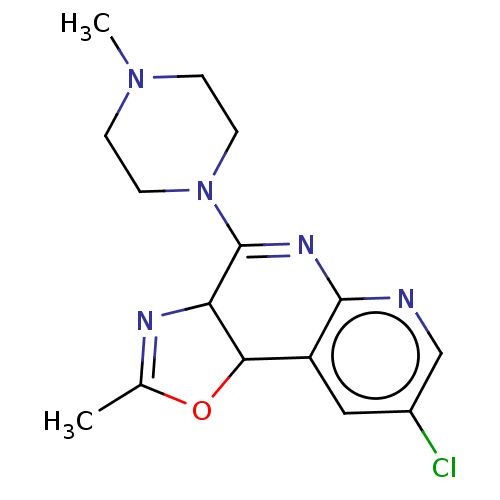 (US9586959, Compound 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294377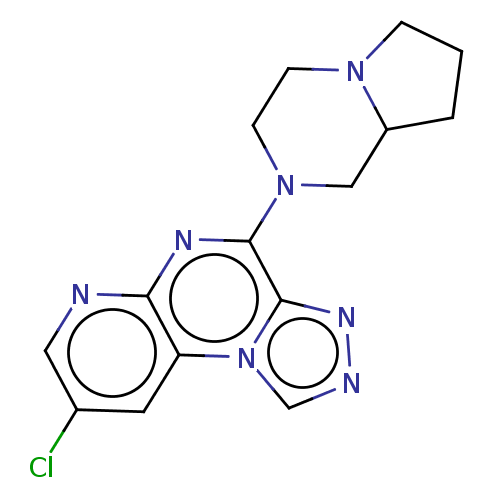 (US9586959, Compound 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294368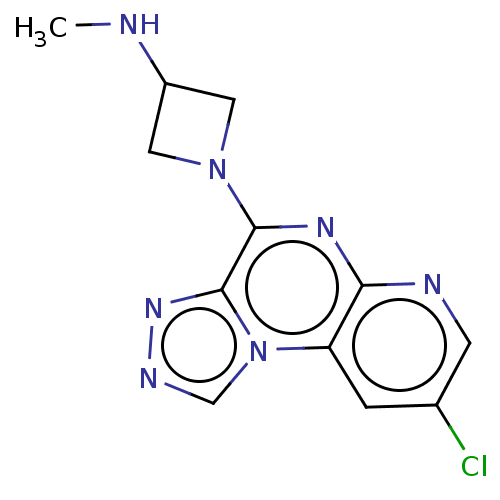 (US9586959, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294408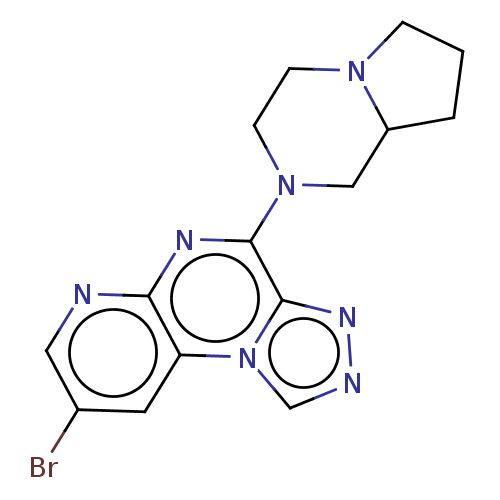 (US9586959, Compound 74) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294416 (US9586959, Compound 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294409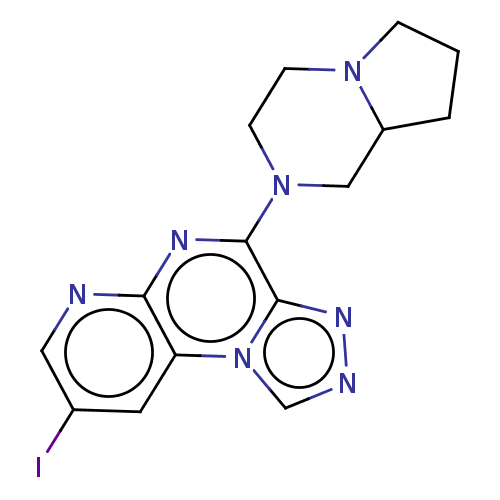 (US9586959, Compound 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294415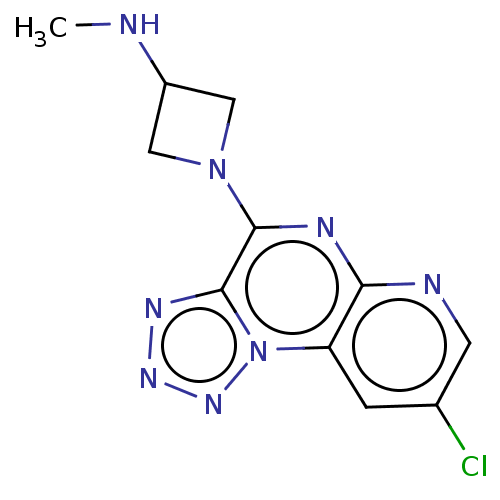 (US9586959, Compound 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294385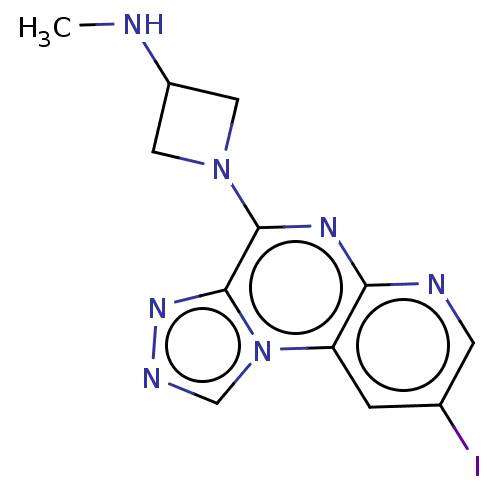 (US9586959, Compound 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294366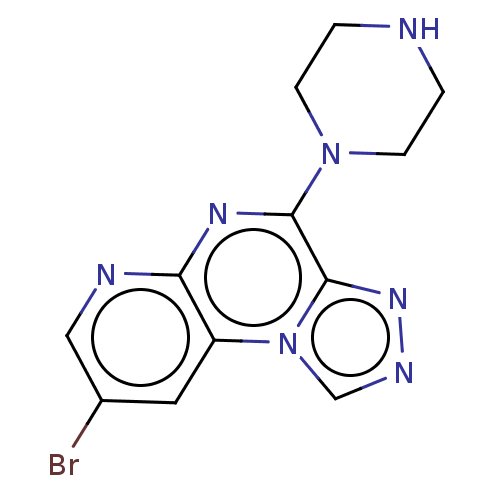 (US9586959, Compound 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294364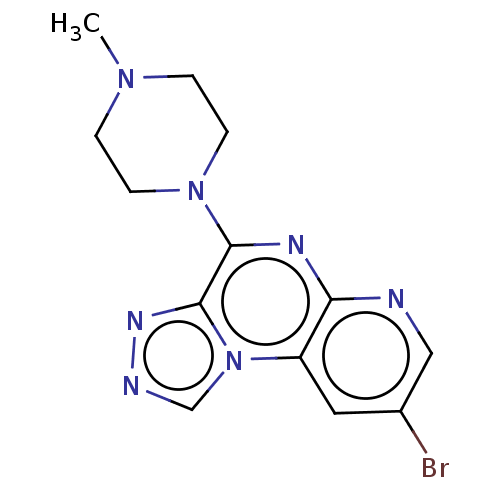 (US9586959, Compound 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294382 (US9586959, Compound 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294379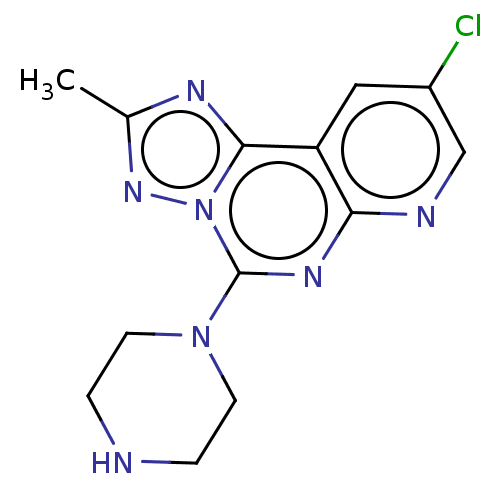 (US9586959, Compound 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294374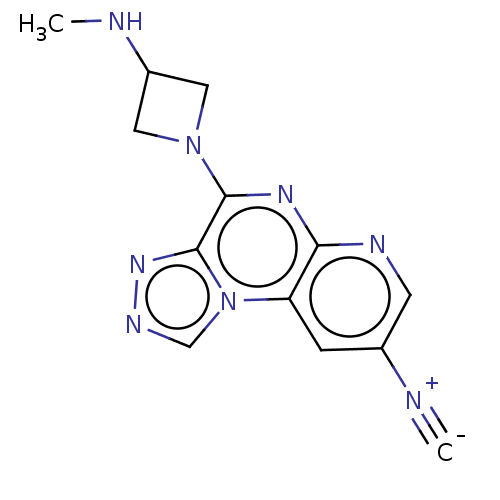 (US9586959, Compound 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294381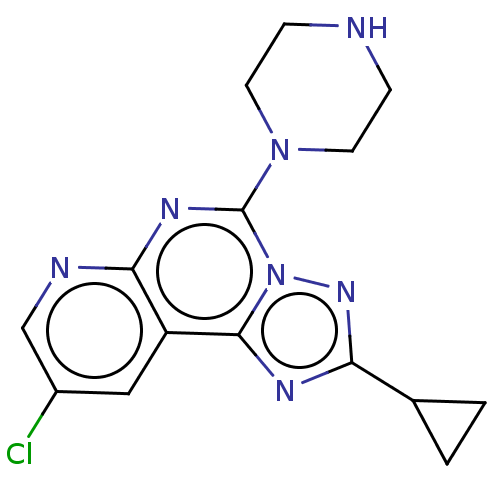 (US9586959, Compound 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294362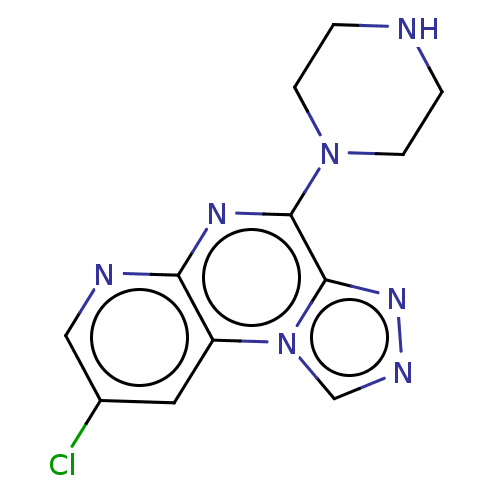 (US9586959, Compound 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 133 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294372 (US9586959, Compound 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294383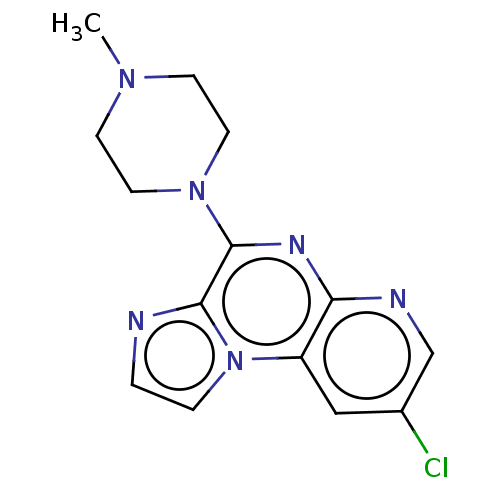 (US9586959, Compound 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 154 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294423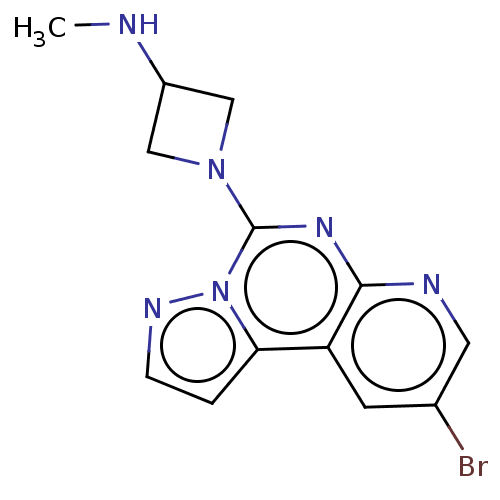 (US9586959, Compound 110) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294380 (US9586959, Compound 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294367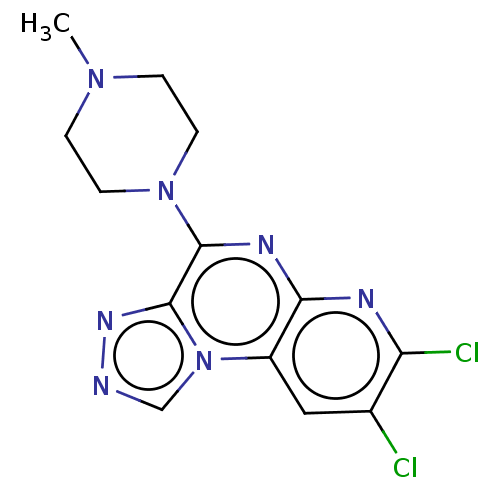 (US9586959, Compound 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 177 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294387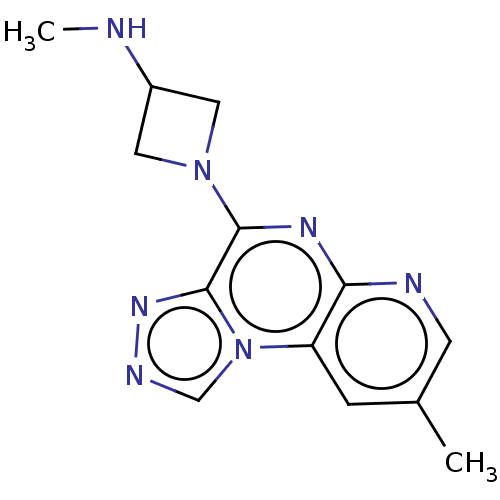 (US9586959, Compound 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294391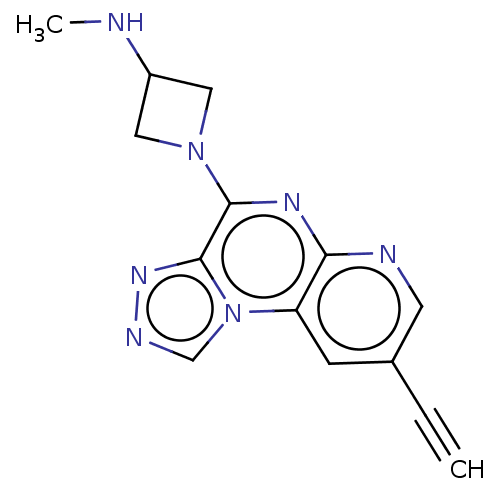 (US9586959, Compound 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294393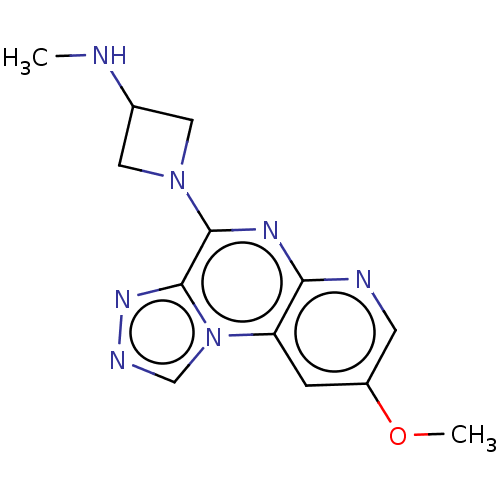 (US9586959, Compound 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294426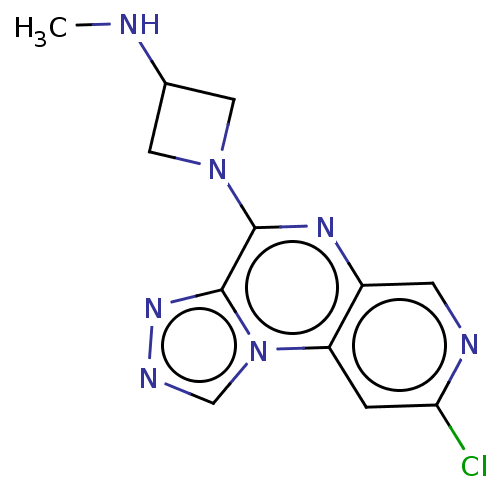 (US9586959, Compound 117) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294389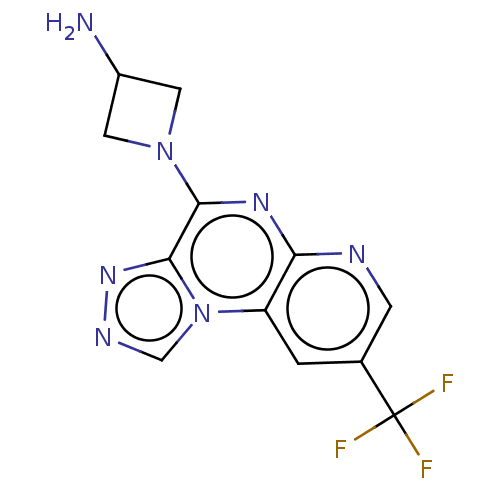 (US9586959, Compound 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294375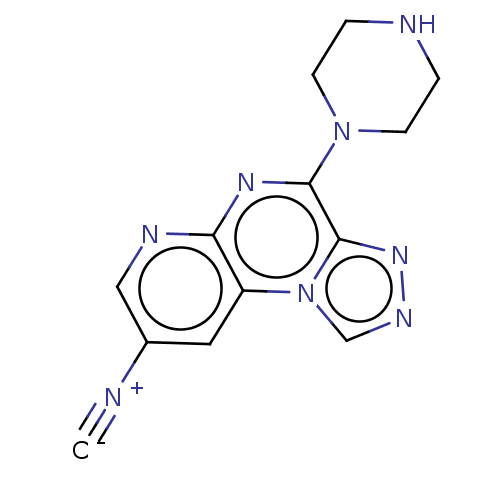 (US9586959, Compound 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294420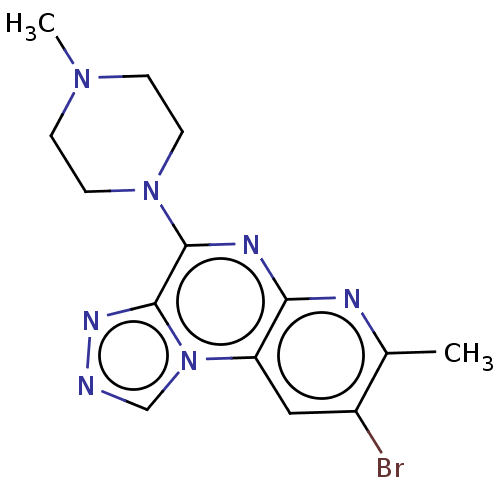 (US9586959, Compound 100) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 231 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294365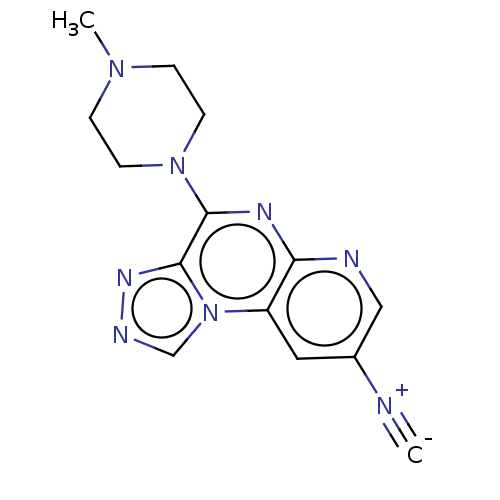 (US9586959, Compound 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 238 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294398 (US9586959, Compound 64) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294425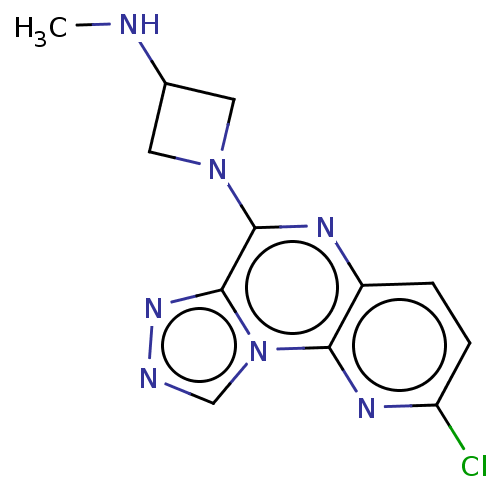 (US9586959, Compound 116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294424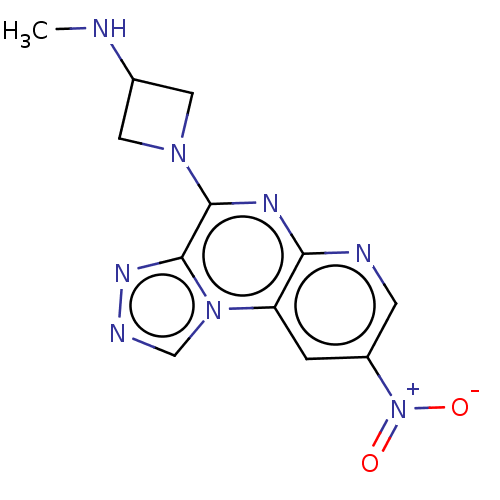 (US9586959, Compound 111) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294412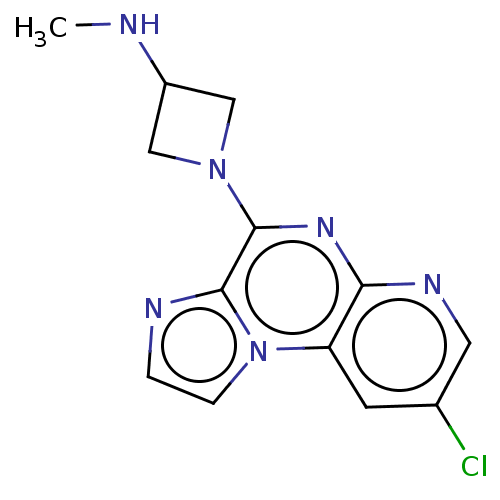 (US9586959, Compound 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294392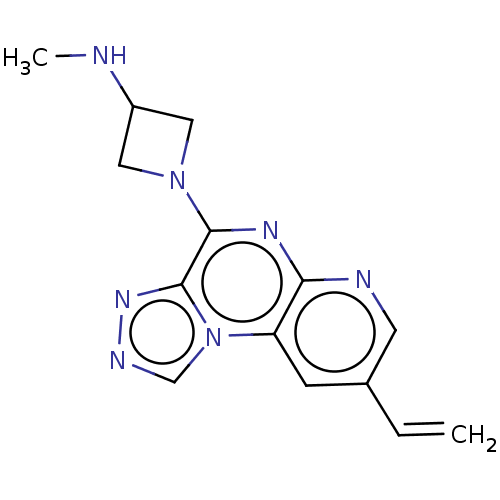 (US9586959, Compound 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294371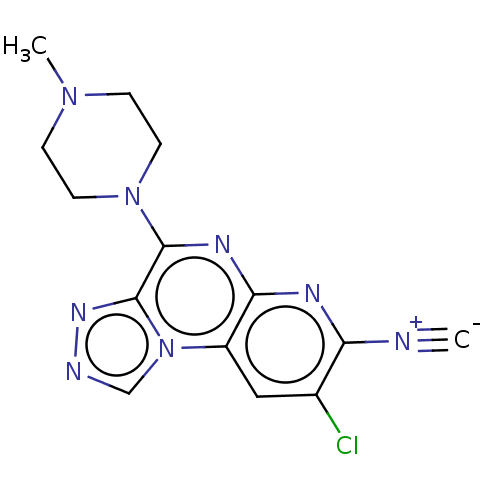 (US9586959, Compound 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294390 (US9586959, Compound 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294401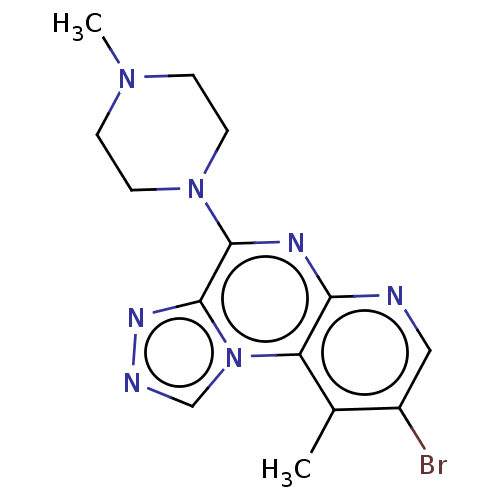 (US9586959, Compound 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294405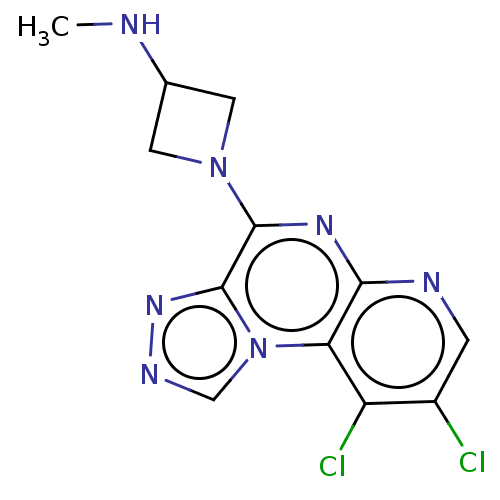 (US9586959, Compound 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 314 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294413 (US9586959, Compound 89) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294370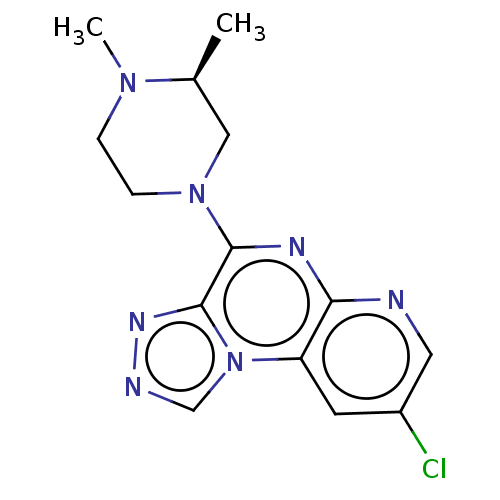 (US9586959, Compound 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294411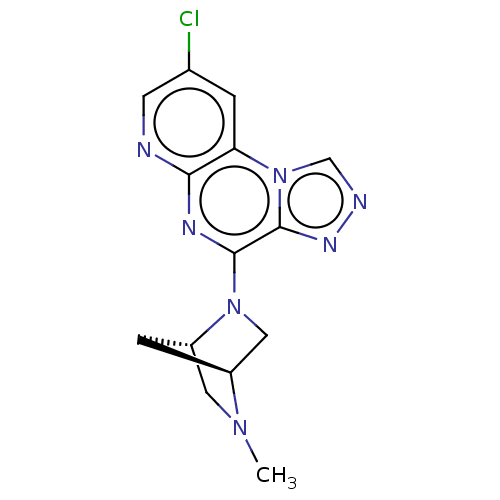 (US9586959, Compound 78) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294376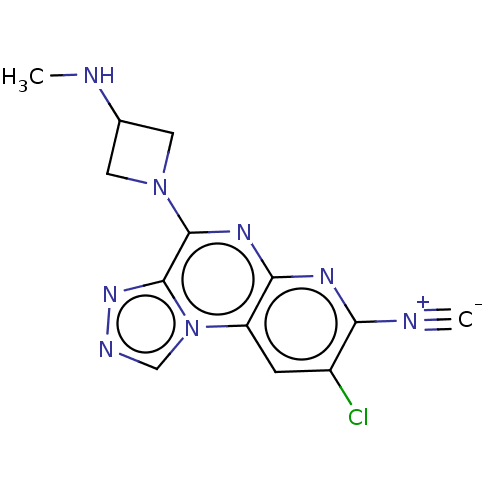 (US9586959, Compound 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294410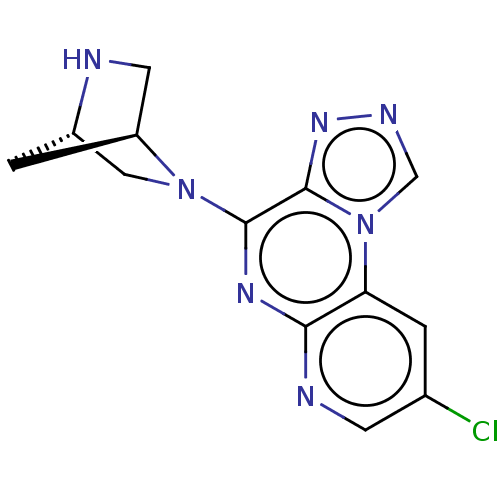 (US9586959, Compound 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294360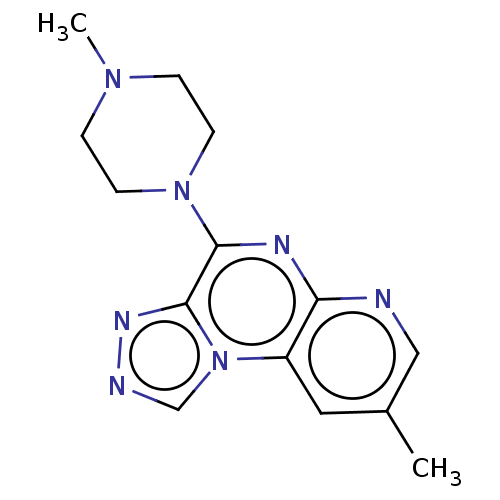 (US9586959, Compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 506 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294396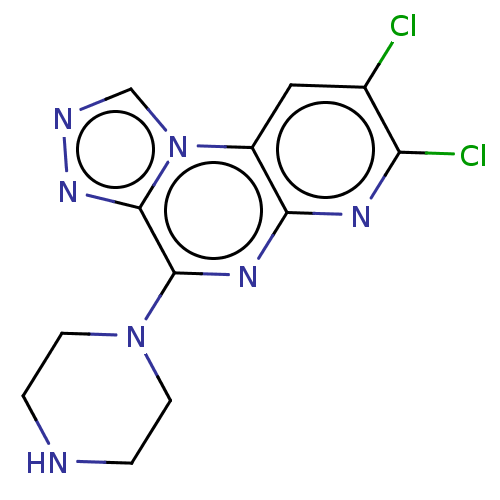 (US9586959, Compound 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294397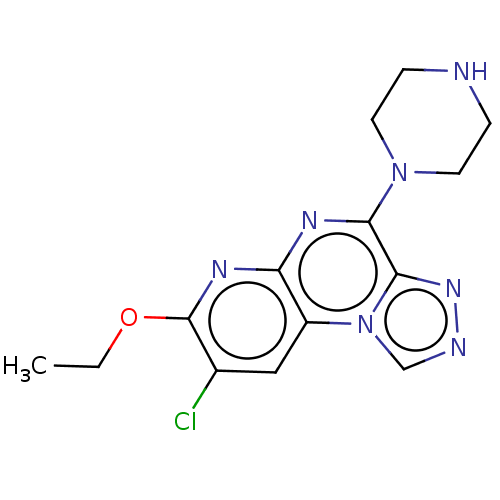 (US9586959, Compound 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294395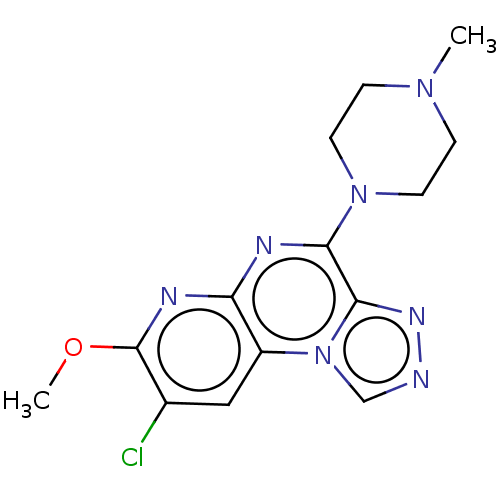 (US9586959, Compound 60) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 610 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294384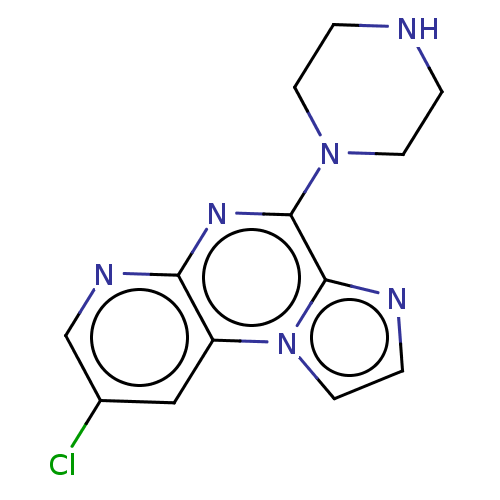 (US9586959, Compound 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 618 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294422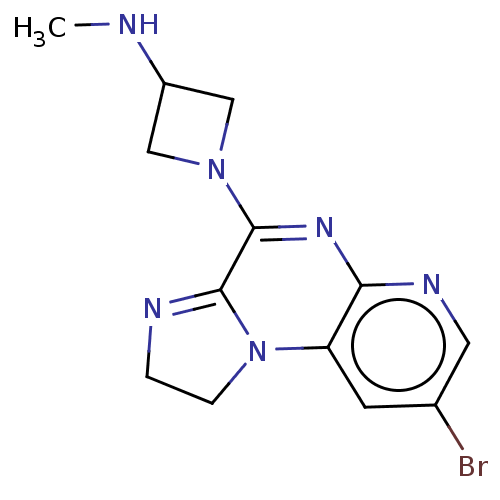 (US9586959, Compound 107) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294388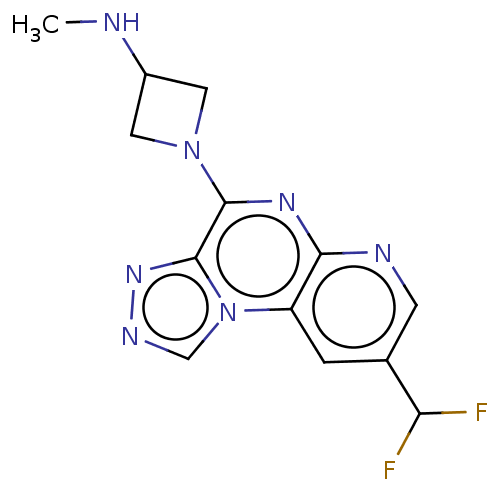 (US9586959, Compound 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 710 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294400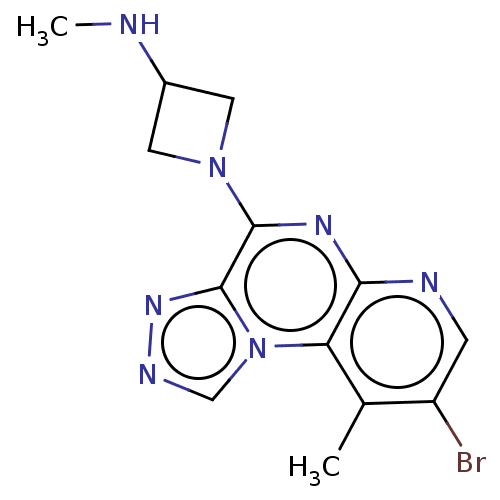 (US9586959, Compound 65) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 730 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294369 (US9586959, Compound 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294394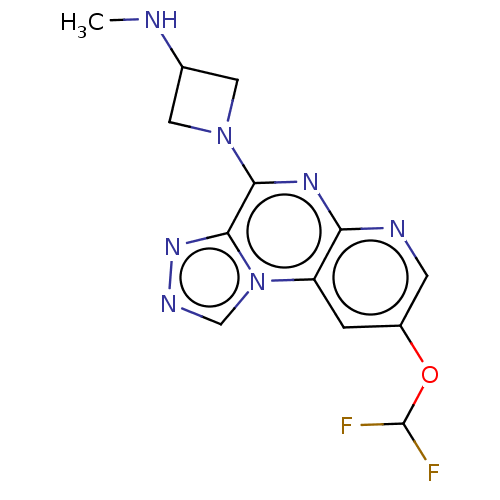 (US9586959, Compound 59) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294368 (US9586959, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
C&C RESEARCH LABORATORIES US Patent | Assay Description The binding assays of the human serotonin 3 receptor for present invention were performed at Cerep (Poitiers, France). | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294427 (US9586959, Compound disclosed in WO 2010030785, Ex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
C&C RESEARCH LABORATORIES US Patent | Assay Description The binding assays of the human serotonin 3 receptor for present invention were performed at Cerep (Poitiers, France). | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294466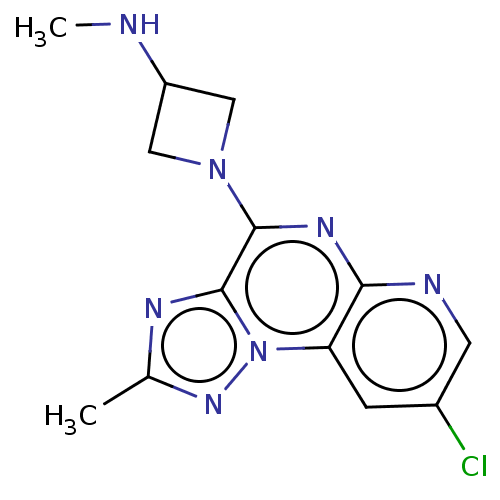 (US9586959, Compound 97) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294361 (US9586959, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294382 (US9586959, Compound 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
C&C RESEARCH LABORATORIES US Patent | Assay Description The binding assays of the human serotonin 3 receptor for present invention were performed at Cerep (Poitiers, France). | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294374 (US9586959, Compound 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
C&C RESEARCH LABORATORIES US Patent | Assay Description The binding assays of the human serotonin 3 receptor for present invention were performed at Cerep (Poitiers, France). | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294415 (US9586959, Compound 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
C&C RESEARCH LABORATORIES US Patent | Assay Description The binding assays of the human serotonin 3 receptor for present invention were performed at Cerep (Poitiers, France). | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM294385 (US9586959, Compound 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
C&C RESEARCH LABORATORIES US Patent | Assay Description The binding assays of the human serotonin 3 receptor for present invention were performed at Cerep (Poitiers, France). | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294423 (US9586959, Compound 110) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294474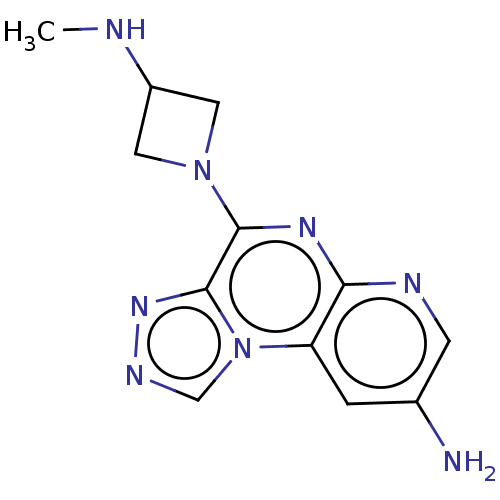 (US9586959, Compound 112) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294370 (US9586959, Compound 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294372 (US9586959, Compound 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294442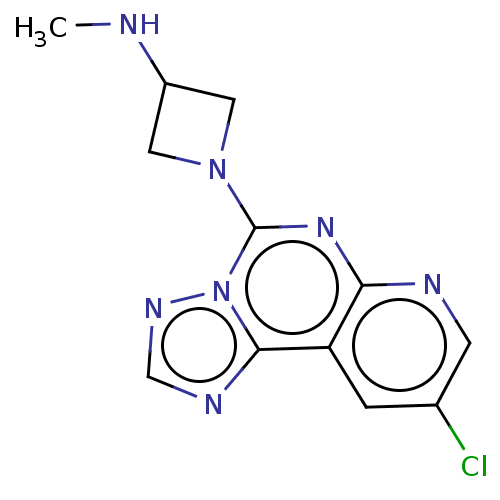 (US9586959, Compound 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294391 (US9586959, Compound 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294451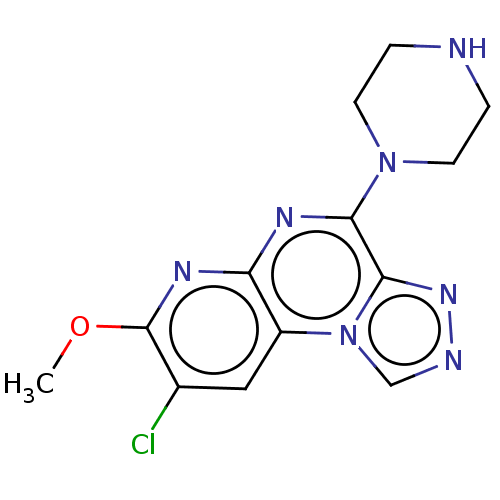 (US9586959, Compound 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294459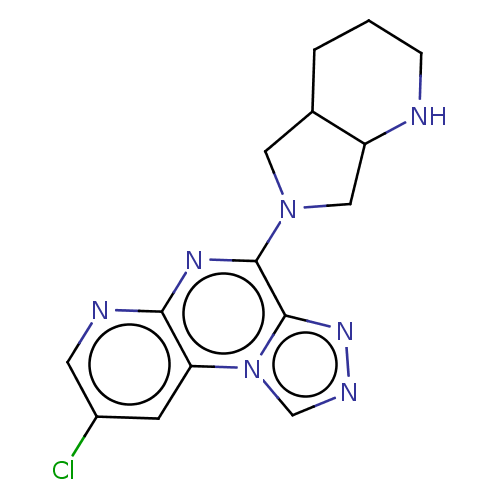 (US9586959, Compound 82) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294461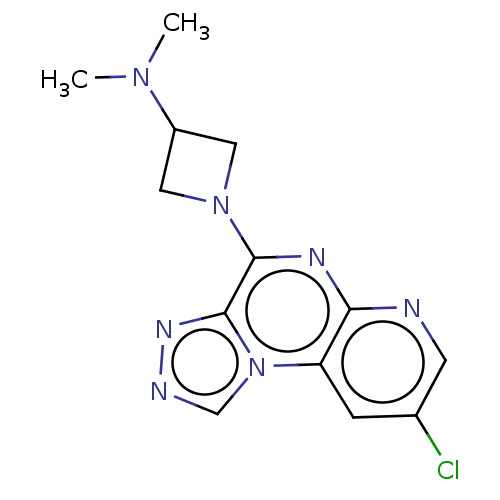 (US9586959, Compound 84) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294420 (US9586959, Compound 100) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294468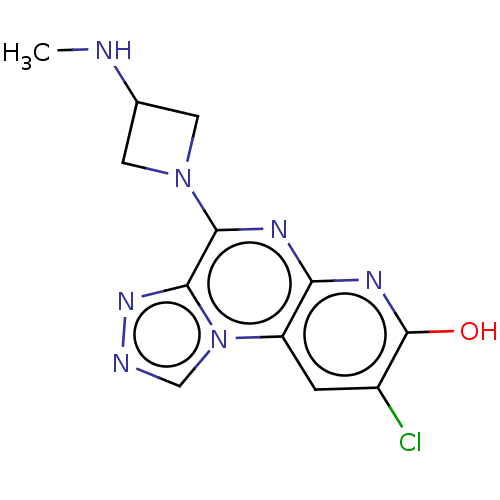 (US9586959, Compound 101) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294476 (US9586959, Compound 114) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294428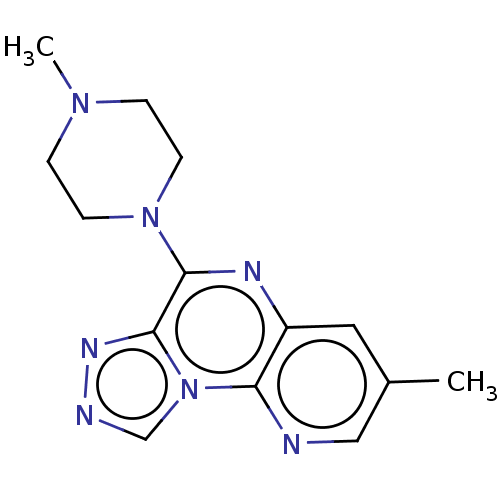 (US9586959, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294431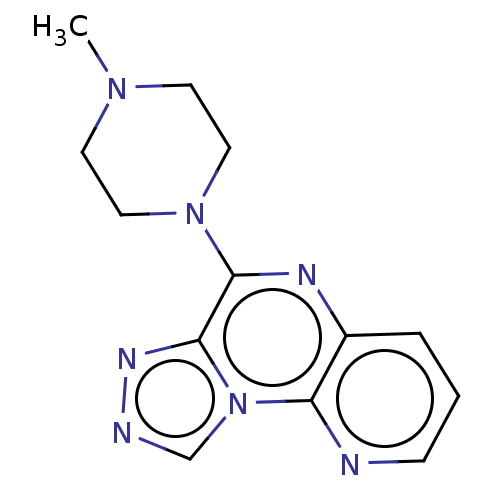 (US9586959, Compound 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294365 (US9586959, Compound 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294368 (US9586959, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294369 (US9586959, Compound 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294379 (US9586959, Compound 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294443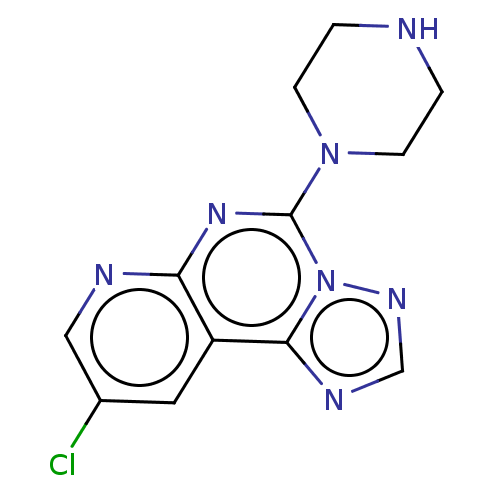 (US9586959, Compound 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294445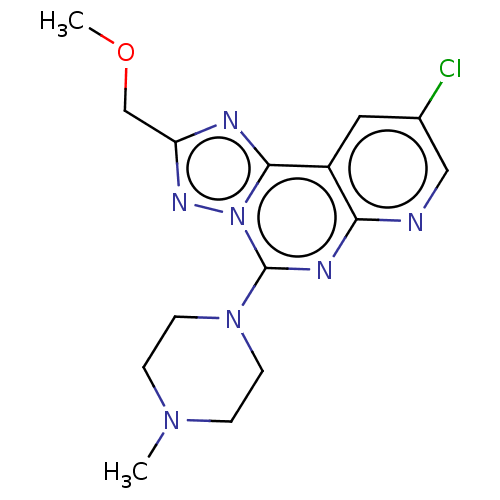 (US9586959, Compound 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294449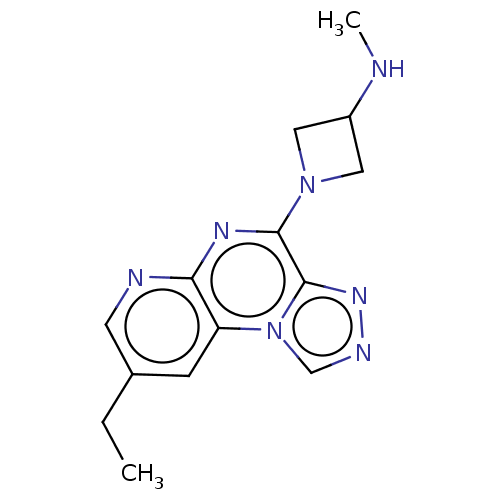 (US9586959, Compound 56) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294397 (US9586959, Compound 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294462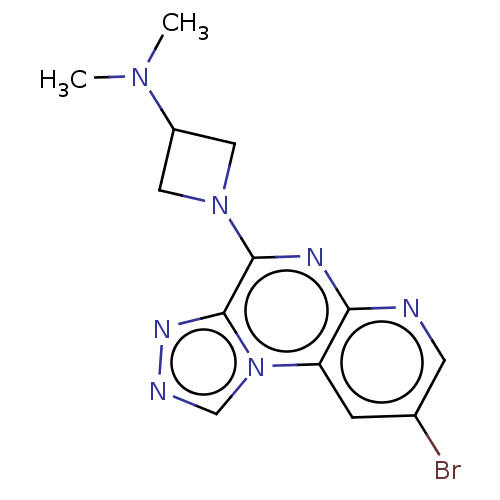 (US9586959, Compound 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294463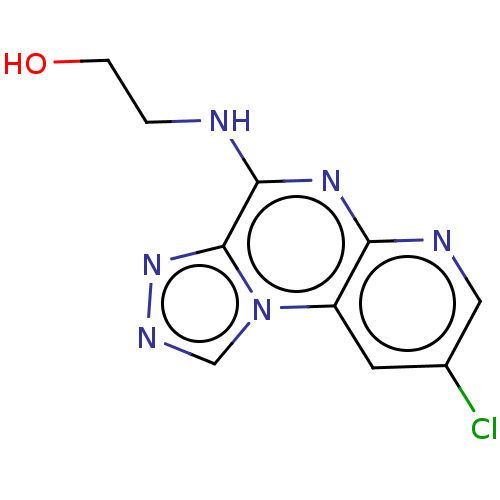 (US9586959, Compound 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294465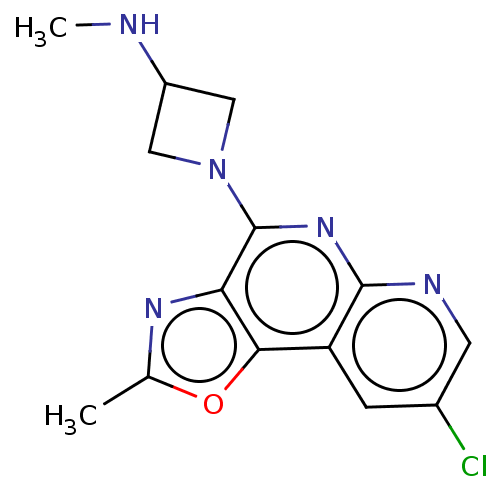 (US9586959, Compound 91) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294415 (US9586959, Compound 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294469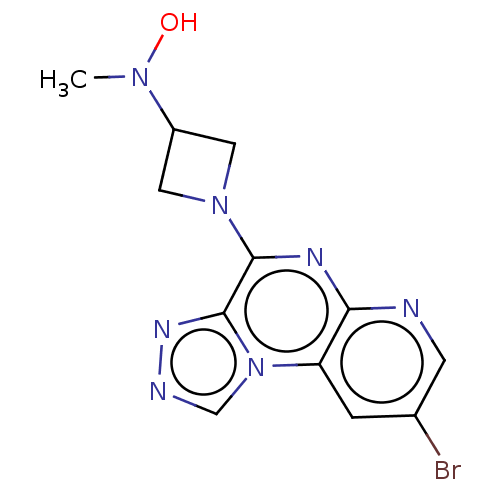 (US9586959, Compound 102) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294473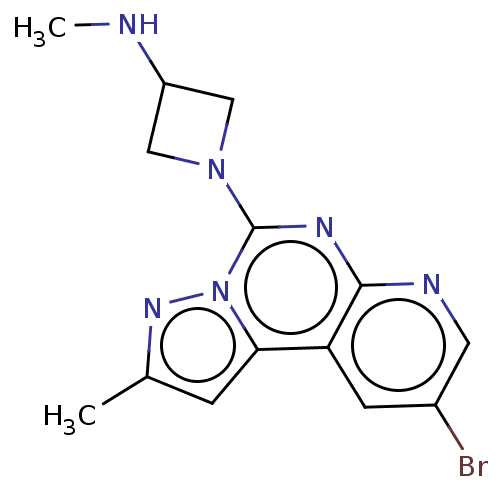 (US9586959, Compound 109) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294424 (US9586959, Compound 111) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294475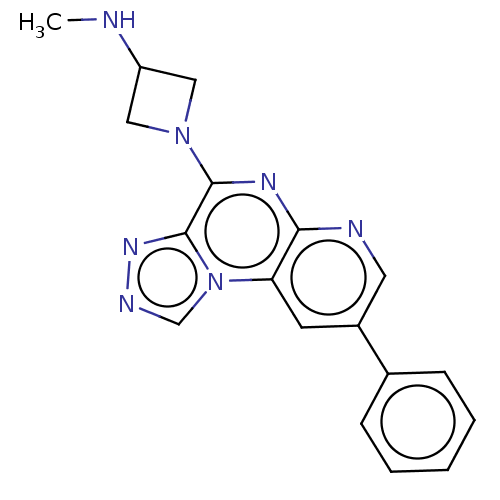 (US9586959, Compound 113) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294430 (US9586959, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294367 (US9586959, Compound 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294439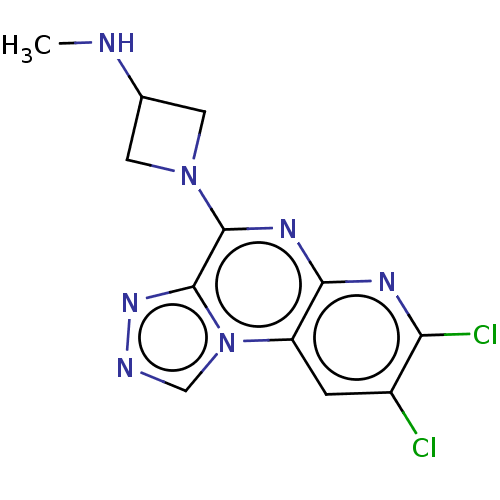 (US9586959, Compound 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294378 (US9586959, Compound 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294441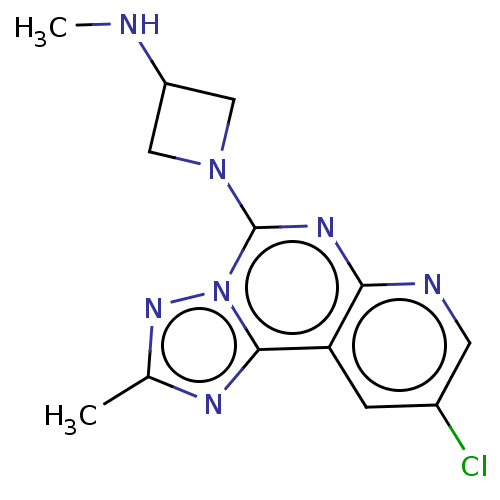 (US9586959, Compound 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294444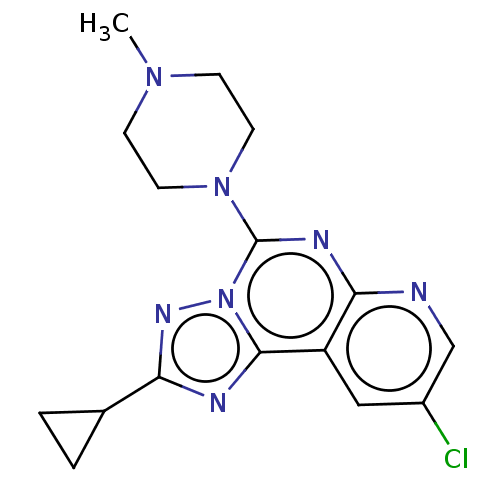 (US9586959, Compound 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294448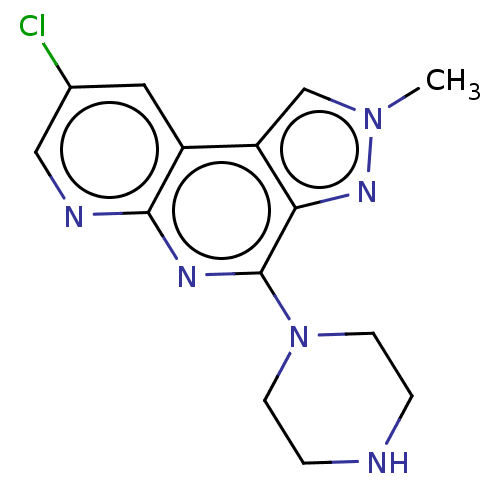 (US9586959, Compound 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294385 (US9586959, Compound 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294387 (US9586959, Compound 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294393 (US9586959, Compound 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294452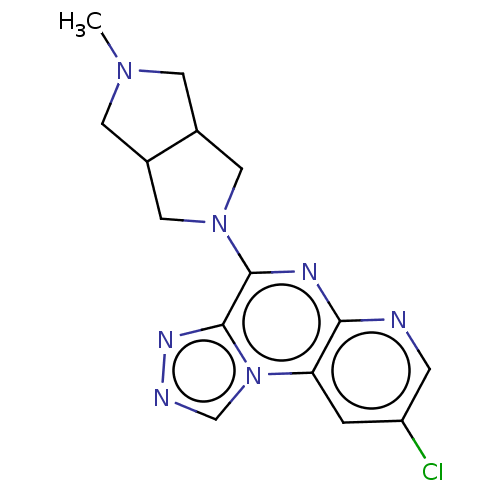 (US9586959, Compound 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294460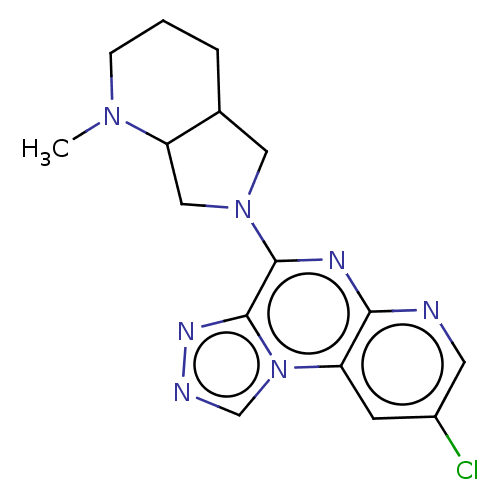 (US9586959, Compound 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294422 (US9586959, Compound 107) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294432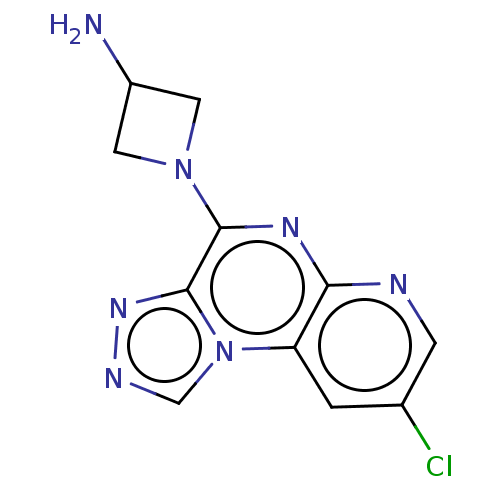 (US9586959, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294434 (US9586959, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294440 (US9586959, Compound 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294386 (US9586959, Compound 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294389 (US9586959, Compound 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294392 (US9586959, Compound 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294396 (US9586959, Compound 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294400 (US9586959, Compound 65) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294453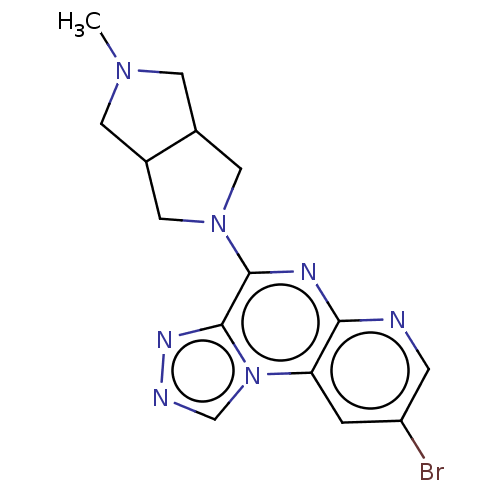 (US9586959, Compound 71) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294456 (US9586959, Compound 76) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294411 (US9586959, Compound 78) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294425 (US9586959, Compound 116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294362 (US9586959, Compound 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294433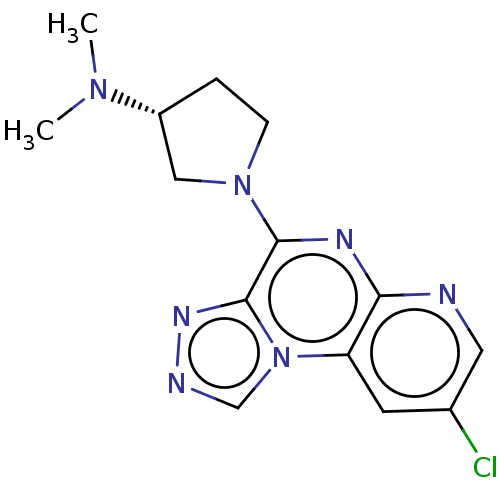 (US9586959, Compound 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294436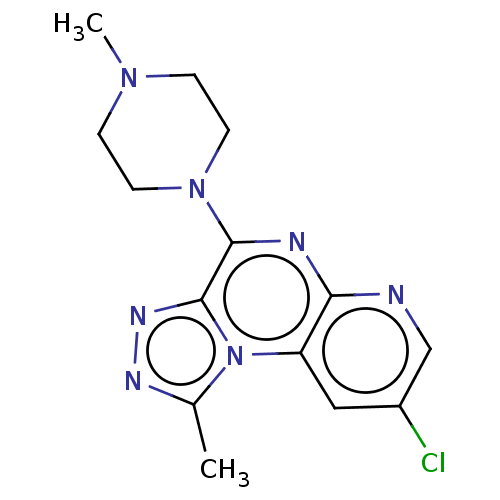 (US9586959, Compound 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294366 (US9586959, Compound 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294450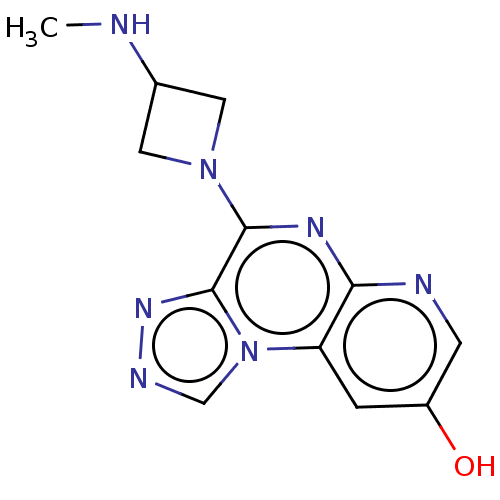 (US9586959, Compound 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294455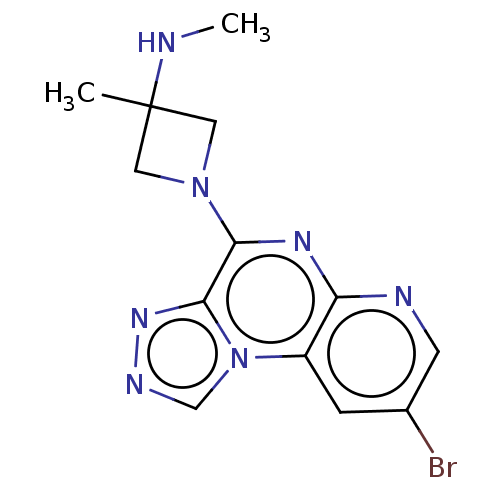 (US9586959, Compound 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294410 (US9586959, Compound 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294412 (US9586959, Compound 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294413 (US9586959, Compound 89) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294464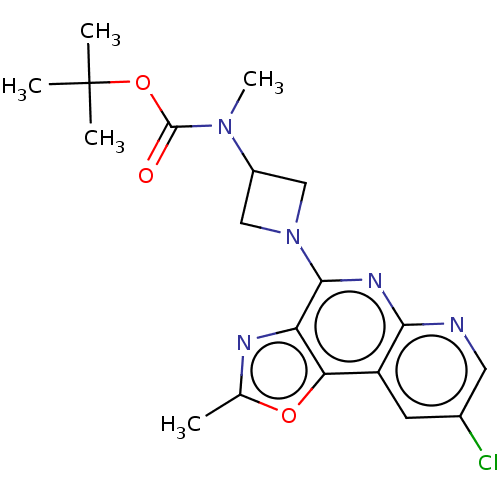 (US9586959, Compound 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294471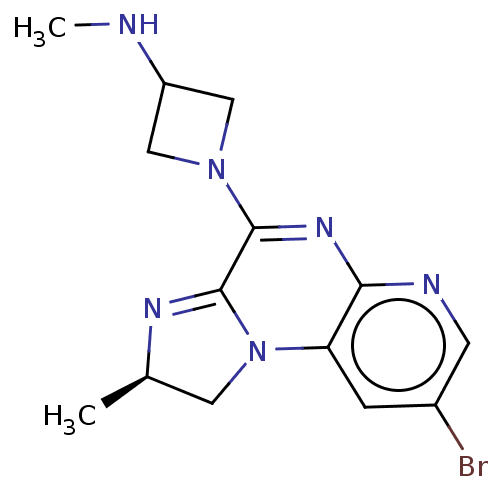 (US9586959, Compound 106) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294477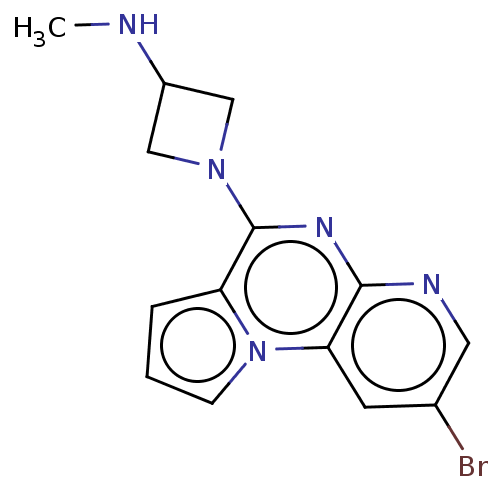 (US9586959, Compound 115) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294360 (US9586959, Compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294438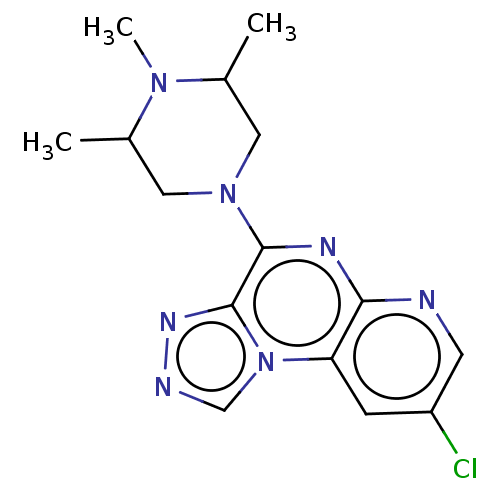 (US9586959, Compound 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294374 (US9586959, Compound 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294446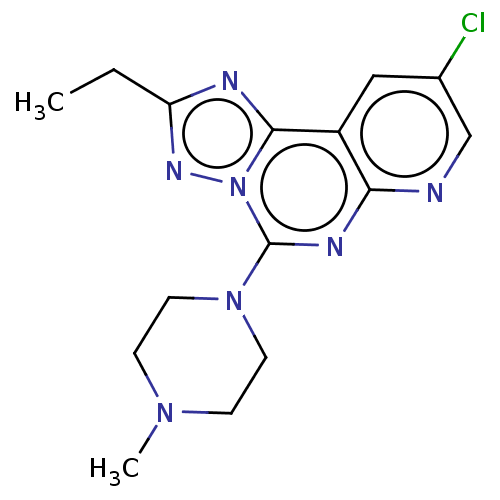 (US9586959, Compound 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294388 (US9586959, Compound 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294394 (US9586959, Compound 59) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294405 (US9586959, Compound 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294457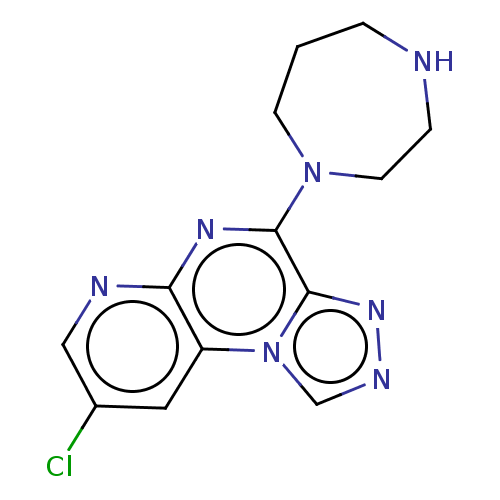 (US9586959, Compound 79) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294414 (US9586959, Compound 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294470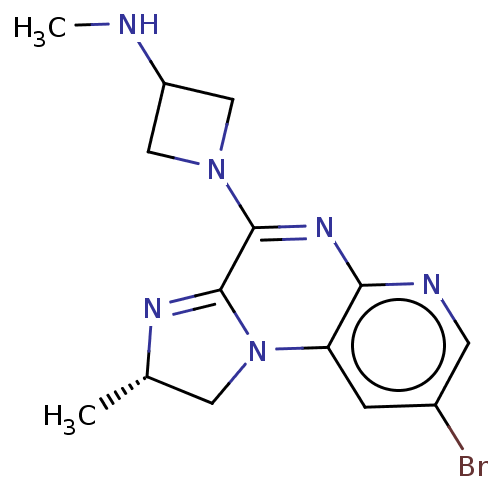 (US9586959, Compound 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294426 (US9586959, Compound 117) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294447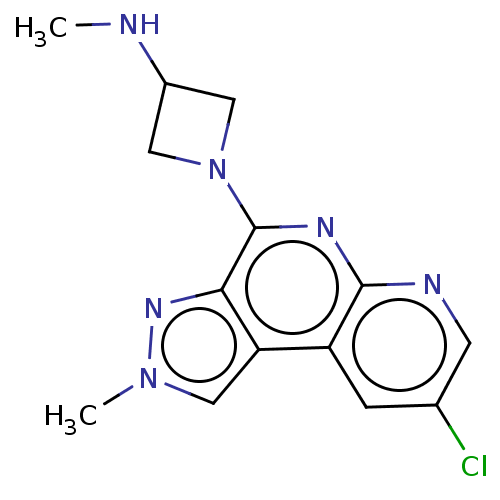 (US9586959, Compound 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294395 (US9586959, Compound 60) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294454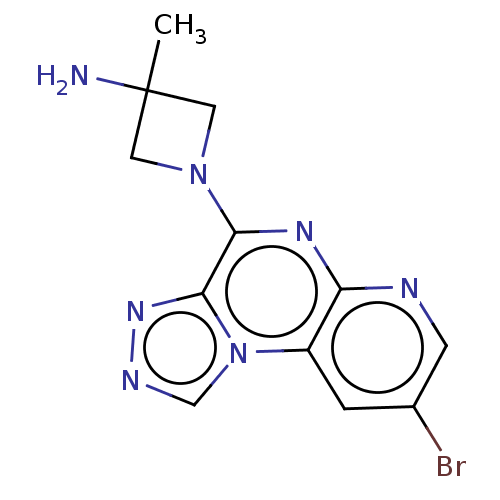 (US9586959, Compound 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294467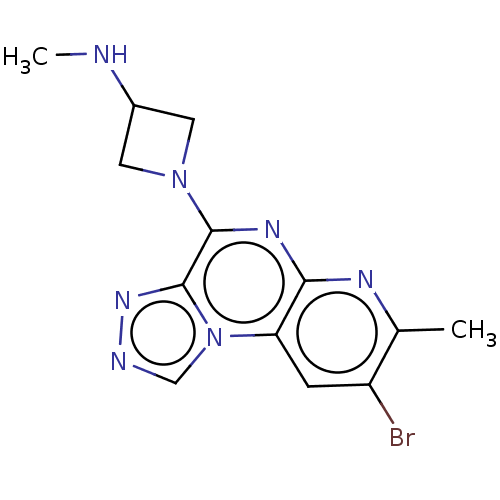 (US9586959, Compound 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294429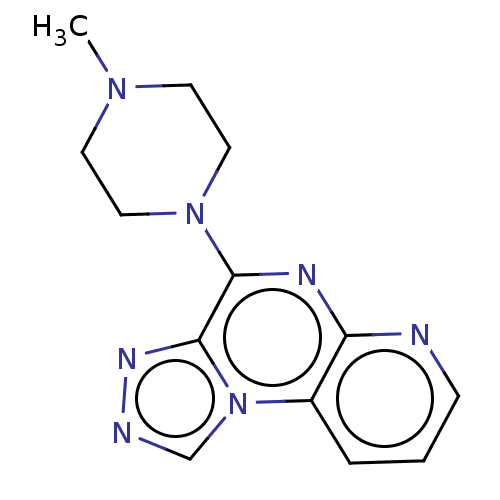 (US9586959, Compound 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294435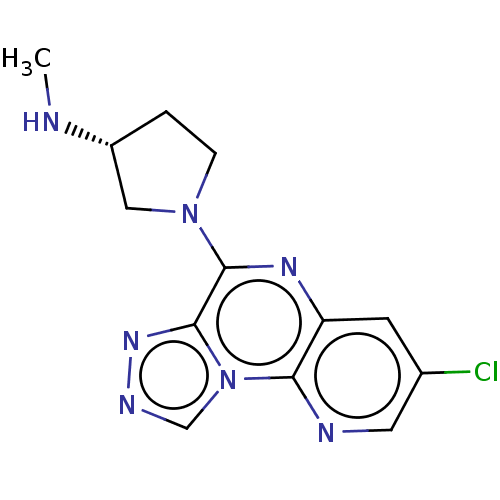 (US9586959, Compound 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294437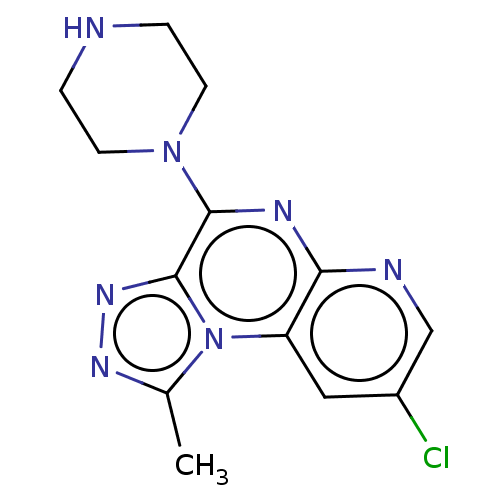 (US9586959, Compound 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294375 (US9586959, Compound 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294376 (US9586959, Compound 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294381 (US9586959, Compound 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294398 (US9586959, Compound 64) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294458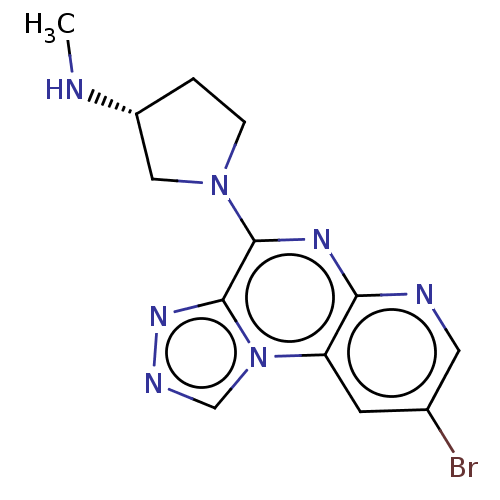 (US9586959, Compound 81) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294472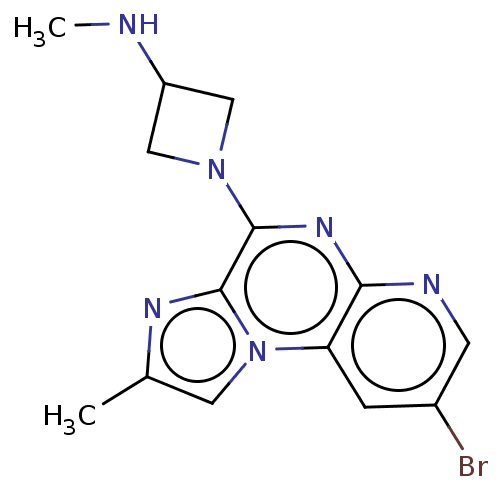 (US9586959, Compound 108) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||