| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50536193 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1933089 (CHEMBL4478741) |
|---|
| IC50 | 100±n/a nM |
|---|
| Citation |  Norcross, NR; Baragaña, B; Wilson, C; Hallyburton, I; Osuna-Cabello, M; Norval, S; Riley, J; Stojanovski, L; Simeons, FR; Porzelle, A; Grimaldi, R; Wittlin, S; Duffy, S; Avery, VM; Meister, S; Sanz, L; Jiménez-Díaz, B; Angulo-Barturen, I; Ferrer, S; Martínez, MS; Gamo, FJ; Frearson, JA; Gray, DW; Fairlamb, AH; Winzeler, EA; Waterson, D; Campbell, SF; Willis, P; Read, KD; Gilbert, IH Trisubstituted Pyrimidines as Efficacious and Fast-Acting Antimalarials. J Med Chem59:6101-20 (2016) [PubMed] Article Norcross, NR; Baragaña, B; Wilson, C; Hallyburton, I; Osuna-Cabello, M; Norval, S; Riley, J; Stojanovski, L; Simeons, FR; Porzelle, A; Grimaldi, R; Wittlin, S; Duffy, S; Avery, VM; Meister, S; Sanz, L; Jiménez-Díaz, B; Angulo-Barturen, I; Ferrer, S; Martínez, MS; Gamo, FJ; Frearson, JA; Gray, DW; Fairlamb, AH; Winzeler, EA; Waterson, D; Campbell, SF; Willis, P; Read, KD; Gilbert, IH Trisubstituted Pyrimidines as Efficacious and Fast-Acting Antimalarials. J Med Chem59:6101-20 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50536193 |
|---|
| n/a |
|---|
| Name | BDBM50536193 |
|---|
| Synonyms: | CHEMBL548646 | GNF-Pf-1447 | TCMDC-125419 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H19N5 |
|---|
| Mol. Mass. | 365.4305 |
|---|
| SMILES | C1Cc2ccccc2CN1c1cc(nc(n1)-c1ccncc1)-c1cccnc1 |
|---|
| Structure | 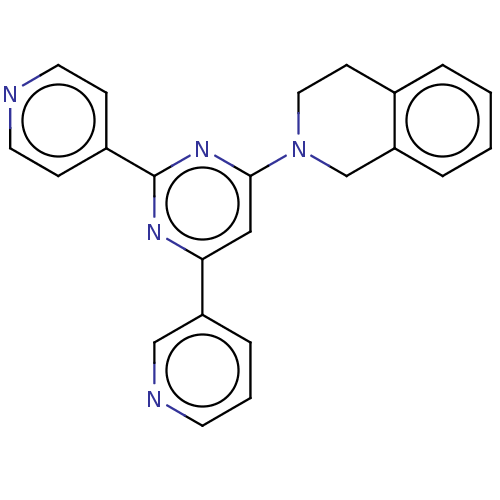
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Norcross, NR; Baragaña, B; Wilson, C; Hallyburton, I; Osuna-Cabello, M; Norval, S; Riley, J; Stojanovski, L; Simeons, FR; Porzelle, A; Grimaldi, R; Wittlin, S; Duffy, S; Avery, VM; Meister, S; Sanz, L; Jiménez-Díaz, B; Angulo-Barturen, I; Ferrer, S; Martínez, MS; Gamo, FJ; Frearson, JA; Gray, DW; Fairlamb, AH; Winzeler, EA; Waterson, D; Campbell, SF; Willis, P; Read, KD; Gilbert, IH Trisubstituted Pyrimidines as Efficacious and Fast-Acting Antimalarials. J Med Chem59:6101-20 (2016) [PubMed] Article
Norcross, NR; Baragaña, B; Wilson, C; Hallyburton, I; Osuna-Cabello, M; Norval, S; Riley, J; Stojanovski, L; Simeons, FR; Porzelle, A; Grimaldi, R; Wittlin, S; Duffy, S; Avery, VM; Meister, S; Sanz, L; Jiménez-Díaz, B; Angulo-Barturen, I; Ferrer, S; Martínez, MS; Gamo, FJ; Frearson, JA; Gray, DW; Fairlamb, AH; Winzeler, EA; Waterson, D; Campbell, SF; Willis, P; Read, KD; Gilbert, IH Trisubstituted Pyrimidines as Efficacious and Fast-Acting Antimalarials. J Med Chem59:6101-20 (2016) [PubMed] Article