 Found 186 hits with Last Name = 'ferrer' and Initial = 's'
Found 186 hits with Last Name = 'ferrer' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50018011
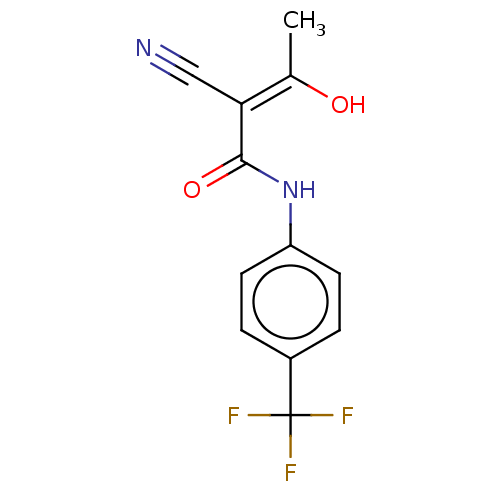
(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536193
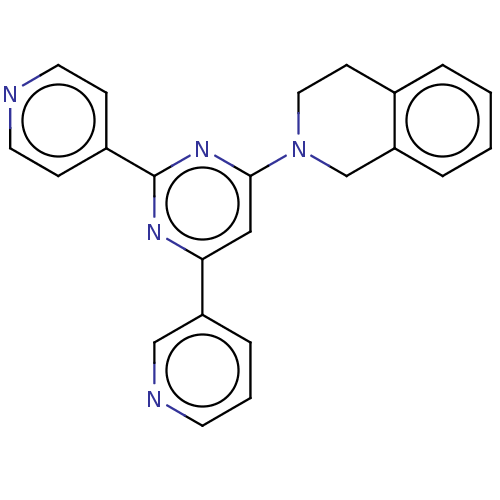
(CHEMBL548646 | GNF-Pf-1447 | TCMDC-125419)Show SMILES C1Cc2ccccc2CN1c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H19N5/c1-2-5-20-16-28(13-9-17(20)4-1)22-14-21(19-6-3-10-25-15-19)26-23(27-22)18-7-11-24-12-8-18/h1-8,10-12,14-15H,9,13,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536194

(CHEMBL4569641)Show SMILES C(C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C24H28N6O/c1-2-21(17-26-7-1)22-16-23(28-24(27-22)20-3-8-25-9-4-20)30-10-5-19(6-11-30)18-29-12-14-31-15-13-29/h1-4,7-9,16-17,19H,5-6,10-15,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50018011

(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50384600
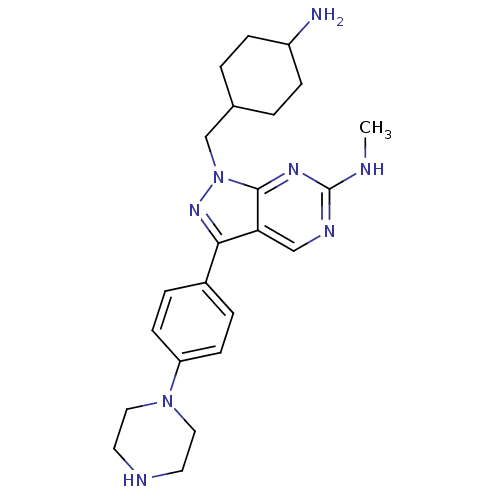
(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218
BindingDB Entry DOI: 10.7270/Q2ZC867R |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218
BindingDB Entry DOI: 10.7270/Q2ZC867R |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50018011

(Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged human DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50030836
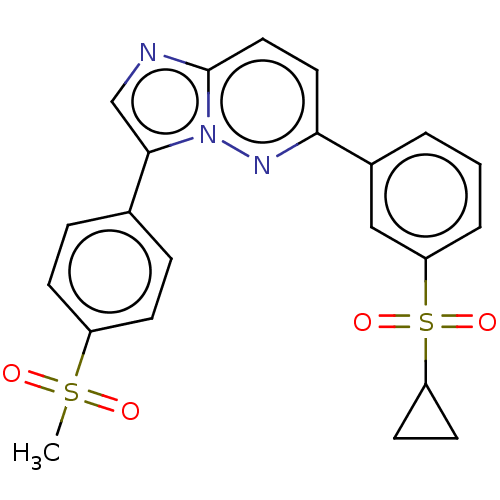
(CHEMBL3355639)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc2ccc(nn12)-c1cccc(c1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19N3O4S2/c1-30(26,27)17-7-5-15(6-8-17)21-14-23-22-12-11-20(24-25(21)22)16-3-2-4-19(13-16)31(28,29)18-9-10-18/h2-8,11-14,18H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by ionworks patch-clamp electrophysiology assay |
J Med Chem 58: 8713-22 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01605
BindingDB Entry DOI: 10.7270/Q24F1SJD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50129589

(CHEMBL3628223)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnn2ccc(nc12)-c1cccc(c1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19N3O4S2/c1-30(26,27)17-7-5-15(6-8-17)20-14-23-25-12-11-21(24-22(20)25)16-3-2-4-19(13-16)31(28,29)18-9-10-18/h2-8,11-14,18H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by ionworks patch-clamp electrophysiology assay |
J Med Chem 58: 8713-22 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01605
BindingDB Entry DOI: 10.7270/Q24F1SJD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50536194

(CHEMBL4569641)Show SMILES C(C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C24H28N6O/c1-2-21(17-26-7-1)22-16-23(28-24(27-22)20-3-8-25-9-4-20)30-10-5-19(6-11-30)18-29-12-14-31-15-13-29/h1-4,7-9,16-17,19H,5-6,10-15,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50129559
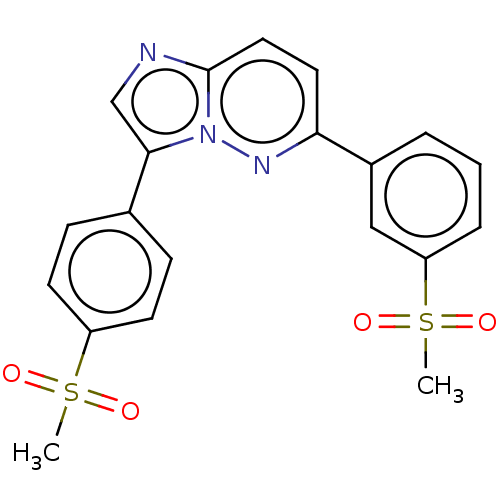
(CHEMBL3234071)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc2ccc(nn12)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C20H17N3O4S2/c1-28(24,25)16-8-6-14(7-9-16)19-13-21-20-11-10-18(22-23(19)20)15-4-3-5-17(12-15)29(2,26)27/h3-13H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by ionworks patch-clamp electrophysiology assay |
J Med Chem 58: 8713-22 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01605
BindingDB Entry DOI: 10.7270/Q24F1SJD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50129562
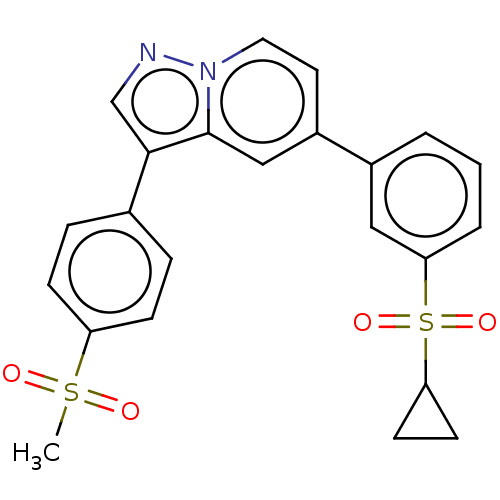
(CHEMBL3628220)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnn2ccc(cc12)-c1cccc(c1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C23H20N2O4S2/c1-30(26,27)19-7-5-16(6-8-19)22-15-24-25-12-11-18(14-23(22)25)17-3-2-4-21(13-17)31(28,29)20-9-10-20/h2-8,11-15,20H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by ionworks patch-clamp electrophysiology assay |
J Med Chem 58: 8713-22 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01605
BindingDB Entry DOI: 10.7270/Q24F1SJD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(t... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558661
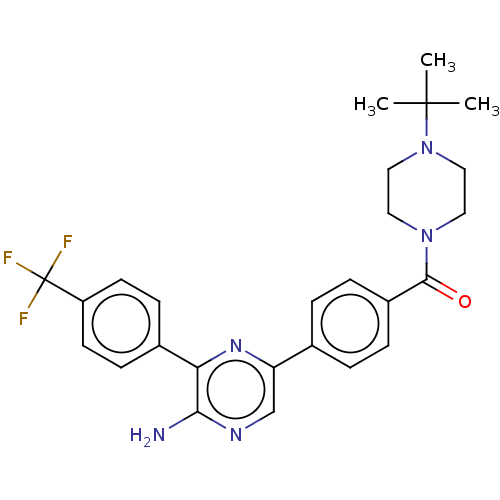
(CHEMBL4763231)Show SMILES CC(C)(C)N1CCN(CC1)C(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50365230

(CHEMBL1956285 | US11903936, Compound DSM265 | US92...)Show SMILES Cc1cc(Nc2ccc(cc2)S(F)(F)(F)(F)F)n2nc(nc2n1)C(C)(F)F Show InChI InChI=1S/C14H12F7N5S/c1-8-7-11(26-13(22-8)24-12(25-26)14(2,15)16)23-9-3-5-10(6-4-9)27(17,18,19,20)21/h3-7,23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099957
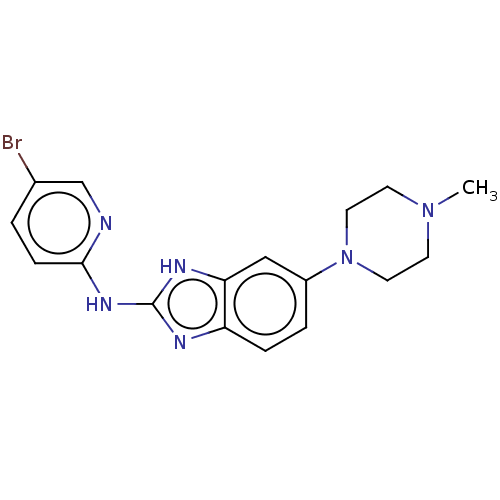
(CHEMBL3321871)Show InChI InChI=1S/C17H19BrN6/c1-23-6-8-24(9-7-23)13-3-4-14-15(10-13)21-17(20-14)22-16-5-2-12(18)11-19-16/h2-5,10-11H,6-9H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50536194

(CHEMBL4569641)Show SMILES C(C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C24H28N6O/c1-2-21(17-26-7-1)22-16-23(28-24(27-22)20-3-8-25-9-4-20)30-10-5-19(6-11-30)18-29-12-14-31-15-13-29/h1-4,7-9,16-17,19H,5-6,10-15,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(t... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020413
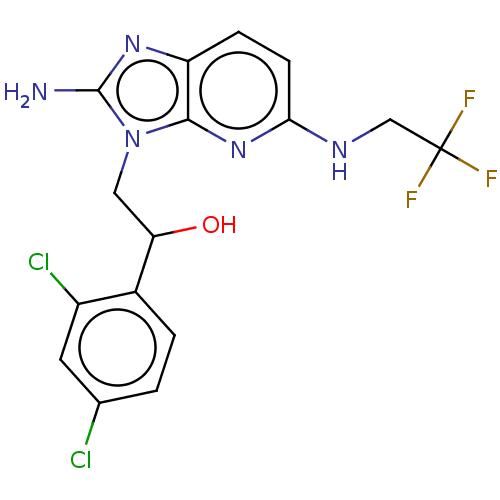
(CHEMBL3289807)Show SMILES Nc1nc2ccc(NCC(F)(F)F)nc2n1CC(O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H14Cl2F3N5O/c17-8-1-2-9(10(18)5-8)12(27)6-26-14-11(24-15(26)22)3-4-13(25-14)23-7-16(19,20)21/h1-5,12,27H,6-7H2,(H2,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100083
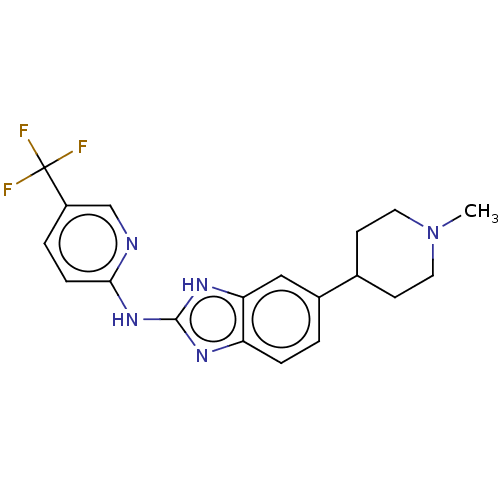
(CHEMBL3321972)Show SMILES CN1CCC(CC1)c1ccc2nc(Nc3ccc(cn3)C(F)(F)F)[nH]c2c1 Show InChI InChI=1S/C19H20F3N5/c1-27-8-6-12(7-9-27)13-2-4-15-16(10-13)25-18(24-15)26-17-5-3-14(11-23-17)19(20,21)22/h2-5,10-12H,6-9H2,1H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50365230

(CHEMBL1956285 | US11903936, Compound DSM265 | US92...)Show SMILES Cc1cc(Nc2ccc(cc2)S(F)(F)(F)(F)F)n2nc(nc2n1)C(C)(F)F Show InChI InChI=1S/C14H12F7N5S/c1-8-7-11(26-13(22-8)24-12(25-26)14(2,15)16)23-9-3-5-10(6-4-9)27(17,18,19,20)21/h3-7,23H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020414

(CHEMBL3289797)Show InChI InChI=1S/C14H15Cl2N3O/c15-9-3-4-10(11(16)5-9)13(20)7-19-6-12(8-1-2-8)18-14(19)17/h3-6,8,13,20H,1-2,7H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50129560

(CHEMBL3628216)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc2ccc(cn12)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C21H18N2O4S2/c1-28(24,25)18-9-6-15(7-10-18)20-13-22-21-11-8-17(14-23(20)21)16-4-3-5-19(12-16)29(2,26)27/h3-14H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of human ERG potassium channel by ionworks patch-clamp electrophysiology assay |
J Med Chem 58: 8713-22 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01605
BindingDB Entry DOI: 10.7270/Q24F1SJD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020415
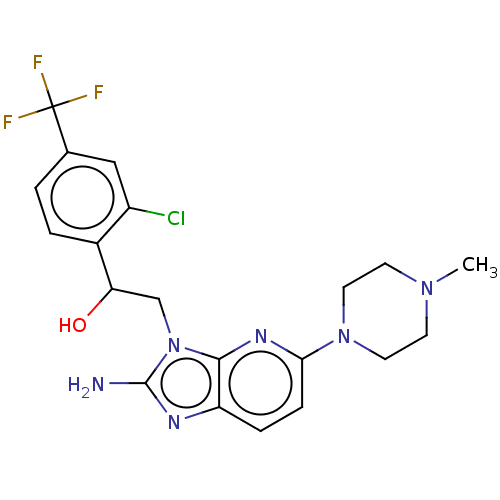
(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558662

(CHEMBL4763365)Show SMILES CN1CCCN(CC1)C(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4/3A5
(Homo sapiens (Human)) | BDBM50538344
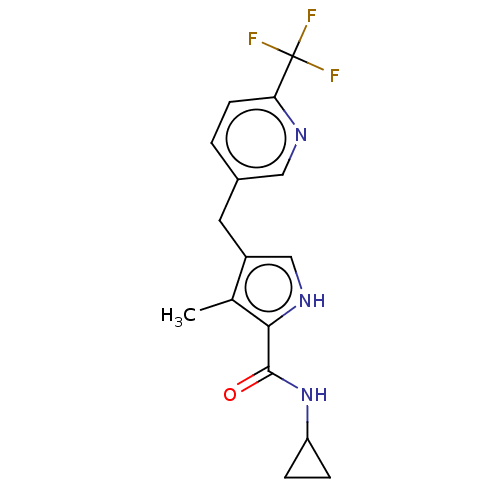
(CHEMBL4633246)Show SMILES Cc1c(Cc2ccc(nc2)C(F)(F)F)c[nH]c1C(=O)NC1CC1 Show InChI InChI=1S/C16H16F3N3O/c1-9-11(8-21-14(9)15(23)22-12-3-4-12)6-10-2-5-13(20-7-10)16(17,18)19/h2,5,7-8,12,21H,3-4,6H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4/5 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by substrate addition and measured af... |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4/3A5
(Homo sapiens (Human)) | BDBM50538344

(CHEMBL4633246)Show SMILES Cc1c(Cc2ccc(nc2)C(F)(F)F)c[nH]c1C(=O)NC1CC1 Show InChI InChI=1S/C16H16F3N3O/c1-9-11(8-21-14(9)15(23)22-12-3-4-12)6-10-2-5-13(20-7-10)16(17,18)19/h2,5,7-8,12,21H,3-4,6H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4/5 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by substrate addition and measured... |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50536191

(CHEMBL4584780)Show SMILES C1CN(CCO1)C1CCN(CC1)c1cc(nc(n1)-c1ccncc1)-c1cccnc1 Show InChI InChI=1S/C23H26N6O/c1-2-19(17-25-7-1)21-16-22(27-23(26-21)18-3-8-24-9-4-18)29-10-5-20(6-11-29)28-12-14-30-15-13-28/h1-4,7-9,16-17,20H,5-6,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 3-Cyano-7-Eth... |
J Med Chem 59: 6101-20 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00028
BindingDB Entry DOI: 10.7270/Q2H41VZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50517286
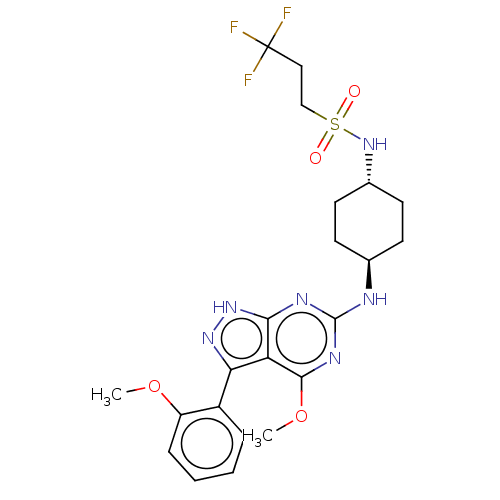
(CHEMBL4516798)Show SMILES COc1ccccc1-c1n[nH]c2nc(N[C@H]3CC[C@@H](CC3)NS(=O)(=O)CCC(F)(F)F)nc(OC)c12 |r,wU:15.15,wD:18.22,(27.63,-17.54,;29.14,-17.85,;30.16,-16.7,;29.67,-15.24,;30.7,-14.08,;32.21,-14.4,;32.69,-15.86,;31.66,-17.01,;32.15,-18.46,;31.24,-19.72,;32.16,-20.97,;33.63,-20.48,;34.96,-21.25,;36.3,-20.48,;37.63,-21.25,;38.96,-20.48,;38.95,-18.94,;40.29,-18.17,;41.62,-18.95,;41.62,-20.49,;40.29,-21.25,;42.96,-18.18,;44.29,-18.95,;45.05,-20.28,;43.51,-20.27,;45.63,-18.18,;46.96,-18.96,;48.29,-18.19,;49.51,-17.49,;49.8,-18.76,;48.56,-16.6,;36.29,-18.93,;34.96,-18.17,;34.95,-16.63,;36.29,-15.86,;33.62,-18.94,)| Show InChI InChI=1S/C22H27F3N6O4S/c1-34-16-6-4-3-5-15(16)18-17-19(30-29-18)27-21(28-20(17)35-2)26-13-7-9-14(10-8-13)31-36(32,33)12-11-22(23,24)25/h3-6,13-14,31H,7-12H2,1-2H3,(H2,26,27,28,29,30)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 20 mins followed by substrate addition and measured afte... |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218
BindingDB Entry DOI: 10.7270/Q2ZC867R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100015

(CHEMBL3321968)Show SMILES CN1CCN(CC1)c1cc(Cl)c2nc(Nc3ccc(Br)cn3)[nH]c2c1 Show InChI InChI=1S/C17H18BrClN6/c1-24-4-6-25(7-5-24)12-8-13(19)16-14(9-12)21-17(23-16)22-15-3-2-11(18)10-20-15/h2-3,8-10H,4-7H2,1H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50100085

(CHEMBL3321980)Show SMILES CN1CCN(CC1)c1cc2[nH]c(Nc3cc(C4CC4)c(F)c(C)n3)nc2cn1 Show InChI InChI=1S/C20H24FN7/c1-12-19(21)14(13-3-4-13)9-17(23-12)26-20-24-15-10-18(22-11-16(15)25-20)28-7-5-27(2)6-8-28/h9-11,13H,3-8H2,1-2H3,(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558663

(CHEMBL4798032)Show SMILES Nc1ncc(nc1-c1ccc(cc1)C(F)(F)F)-c1ccc(cc1)C(=O)N1CCNCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50558659

(CHEMBL4530341)Show SMILES NC1CCN(C1)C(=O)c1ccc(cc1)-c1cnc(N)c(n1)-c1ccc(cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by IonWorks patch clamp electrophysiology method |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M61PZG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020416
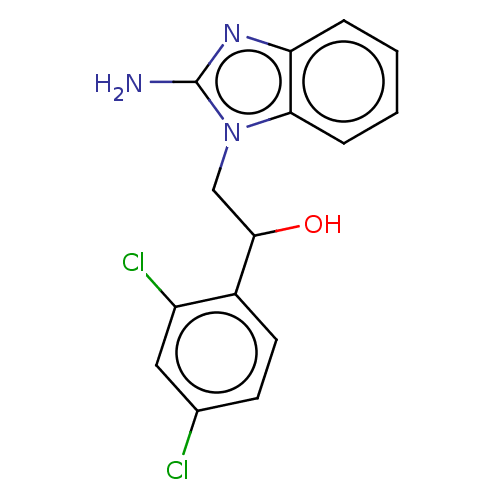
(CHEMBL3289799)Show InChI InChI=1S/C15H13Cl2N3O/c16-9-5-6-10(11(17)7-9)14(21)8-20-13-4-2-1-3-12(13)19-15(20)18/h1-7,14,21H,8H2,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099958

(CHEMBL3321873)Show InChI InChI=1S/C18H22N6/c1-13-3-6-17(19-12-13)22-18-20-15-5-4-14(11-16(15)21-18)24-9-7-23(2)8-10-24/h3-6,11-12H,7-10H2,1-2H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 6642-52 (2014)
Article DOI: 10.1021/jm500715u
BindingDB Entry DOI: 10.7270/Q2377BG1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50517286

(CHEMBL4516798)Show SMILES COc1ccccc1-c1n[nH]c2nc(N[C@H]3CC[C@@H](CC3)NS(=O)(=O)CCC(F)(F)F)nc(OC)c12 |r,wU:15.15,wD:18.22,(27.63,-17.54,;29.14,-17.85,;30.16,-16.7,;29.67,-15.24,;30.7,-14.08,;32.21,-14.4,;32.69,-15.86,;31.66,-17.01,;32.15,-18.46,;31.24,-19.72,;32.16,-20.97,;33.63,-20.48,;34.96,-21.25,;36.3,-20.48,;37.63,-21.25,;38.96,-20.48,;38.95,-18.94,;40.29,-18.17,;41.62,-18.95,;41.62,-20.49,;40.29,-21.25,;42.96,-18.18,;44.29,-18.95,;45.05,-20.28,;43.51,-20.27,;45.63,-18.18,;46.96,-18.96,;48.29,-18.19,;49.51,-17.49,;49.8,-18.76,;48.56,-16.6,;36.29,-18.93,;34.96,-18.17,;34.95,-16.63,;36.29,-15.86,;33.62,-18.94,)| Show InChI InChI=1S/C22H27F3N6O4S/c1-34-16-6-4-3-5-15(16)18-17-19(30-29-18)27-21(28-20(17)35-2)26-13-7-9-14(10-8-13)31-36(32,33)12-11-22(23,24)25/h3-6,13-14,31H,7-12H2,1-2H3,(H2,26,27,28,29,30)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218
BindingDB Entry DOI: 10.7270/Q2ZC867R |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50517285
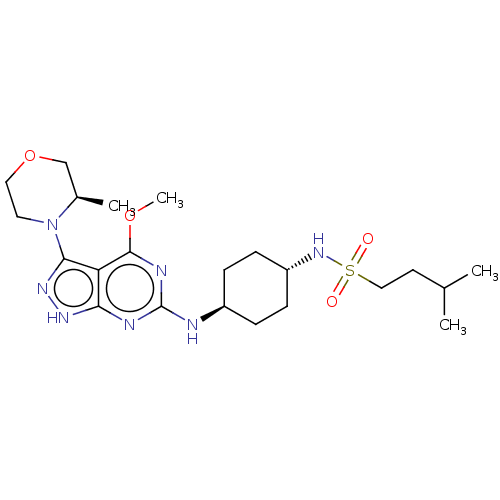
(CHEMBL4572962)Show SMILES COc1nc(N[C@H]2CC[C@@H](CC2)NS(=O)(=O)CCC(C)C)nc2[nH]nc(N3CCOC[C@H]3C)c12 |r,wU:6.5,wD:9.12,31.33,(12.05,-31.41,;10.72,-32.18,;10.72,-33.72,;12.06,-34.49,;12.06,-36.04,;13.4,-36.81,;14.73,-36.03,;14.71,-34.5,;16.05,-33.73,;17.39,-34.5,;17.38,-36.04,;16.05,-36.81,;18.72,-33.73,;20.05,-34.5,;20.81,-35.83,;19.28,-35.83,;21.39,-33.74,;22.72,-34.51,;24.06,-33.75,;25.39,-34.52,;24.06,-32.21,;10.72,-36.81,;9.39,-36.04,;7.92,-36.52,;7.01,-35.27,;7.91,-34.02,;7.43,-32.56,;8.45,-31.43,;7.97,-29.98,;6.47,-29.66,;5.45,-30.81,;5.93,-32.27,;4.91,-33.42,;9.38,-34.49,)| Show InChI InChI=1S/C22H37N7O4S/c1-14(2)9-12-34(30,31)28-17-7-5-16(6-8-17)23-22-24-19-18(21(25-22)32-4)20(27-26-19)29-10-11-33-13-15(29)3/h14-17,28H,5-13H2,1-4H3,(H2,23,24,25,26,27)/t15-,16-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4 by kinobeads-based assay |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218
BindingDB Entry DOI: 10.7270/Q2ZC867R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50538344

(CHEMBL4633246)Show SMILES Cc1c(Cc2ccc(nc2)C(F)(F)F)c[nH]c1C(=O)NC1CC1 Show InChI InChI=1S/C16H16F3N3O/c1-9-11(8-21-14(9)15(23)22-12-3-4-12)6-10-2-5-13(20-7-10)16(17,18)19/h2,5,7-8,12,21H,3-4,6H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate measured for 10 to 30 mins in presence of NADPH regenerating system by U... |
J Med Chem 63: 4929-4956 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00311
BindingDB Entry DOI: 10.7270/Q2M90D6Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data