 Found 1467 hits with Last Name = 'wilson' and Initial = 'c'
Found 1467 hits with Last Name = 'wilson' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Mus musculus) | BDBM50403879
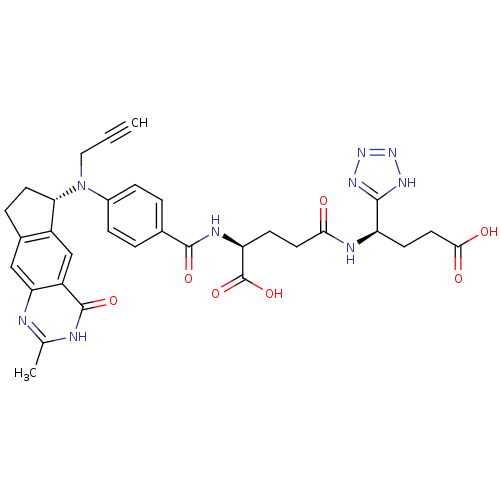
(CHEMBL320217)Show SMILES Cc1nc2cc3CC[C@H](N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)c4nnn[nH]4)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C32H33N9O7/c1-3-14-41(26-11-6-19-15-25-22(16-21(19)26)31(46)34-17(2)33-25)20-7-4-18(5-8-20)30(45)36-24(32(47)48)9-12-27(42)35-23(10-13-28(43)44)29-37-39-40-38-29/h1,4-5,7-8,15-16,23-24,26H,6,9-14H2,2H3,(H,35,42)(H,36,45)(H,43,44)(H,47,48)(H,33,34,46)(H,37,38,39,40)/t23-,24+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50088159
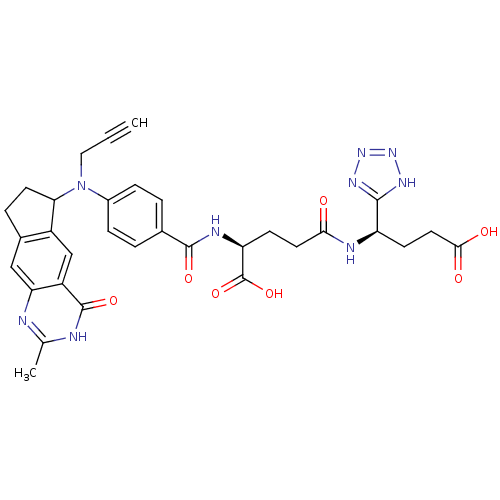
(4-[3-Carboxy-1-(1H-tetrazol-5-yl)-propylcarbamoyl]...)Show SMILES Cc1nc2cc3CCC(N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)c4nnn[nH]4)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C32H33N9O7/c1-3-14-41(26-11-6-19-15-25-22(16-21(19)26)31(46)34-17(2)33-25)20-7-4-18(5-8-20)30(45)36-24(32(47)48)9-12-27(42)35-23(10-13-28(43)44)29-37-39-40-38-29/h1,4-5,7-8,15-16,23-24,26H,6,9-14H2,2H3,(H,35,42)(H,36,45)(H,43,44)(H,47,48)(H,33,34,46)(H,37,38,39,40)/t23-,24+,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442142
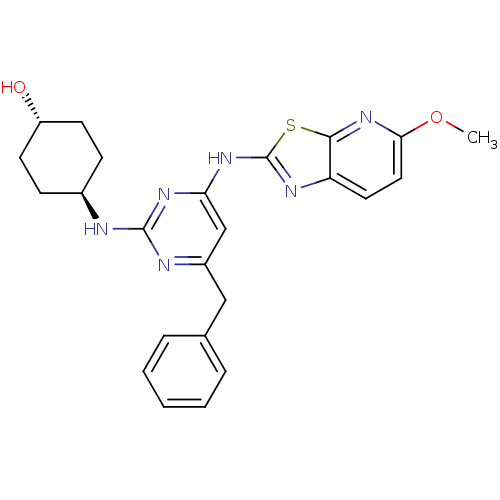
(CHEMBL2441275)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.29,-5.26,;33.51,-3.93,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C24H26N6O2S/c1-32-21-12-11-19-22(30-21)33-24(27-19)29-20-14-17(13-15-5-3-2-4-6-15)26-23(28-20)25-16-7-9-18(31)10-8-16/h2-6,11-12,14,16,18,31H,7-10,13H2,1H3,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442143
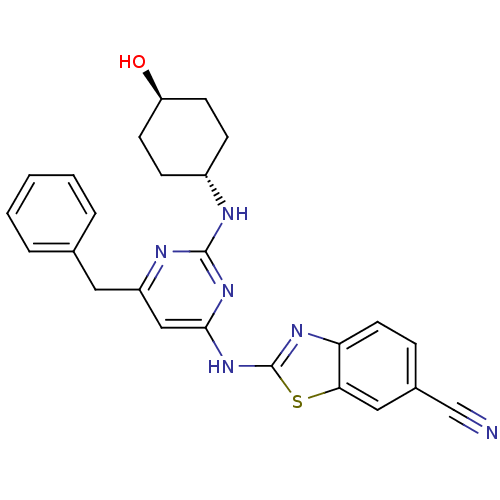
(CHEMBL2441274)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(cc3s2)C#N)n1 |r,wU:1.0,wD:4.7,(23.68,-13.98,;22.35,-14.78,;22.37,-16.32,;21.05,-17.1,;19.71,-16.35,;19.69,-14.81,;21.01,-14.02,;18.39,-17.14,;17.04,-16.39,;15.72,-17.18,;14.38,-16.44,;13.04,-17.23,;11.71,-16.46,;11.71,-14.92,;10.38,-14.14,;9.04,-14.92,;9.04,-16.46,;10.38,-17.22,;14.35,-14.9,;15.67,-14.1,;15.65,-12.56,;16.85,-11.63,;16.82,-10.09,;18.27,-9.58,;18.87,-8.16,;20.4,-7.96,;21.33,-9.19,;20.73,-10.61,;19.2,-10.8,;18.33,-12.08,;22.85,-8.99,;24.37,-8.8,;17.02,-14.86,)| Show InChI InChI=1S/C25H24N6OS/c26-15-17-6-11-21-22(13-17)33-25(29-21)31-23-14-19(12-16-4-2-1-3-5-16)28-24(30-23)27-18-7-9-20(32)10-8-18/h1-6,11,13-14,18,20,32H,7-10,12H2,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442141

(CHEMBL2441276)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3cccnc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C23H24N6OS/c30-18-10-8-16(9-11-18)25-22-26-17(13-15-5-2-1-3-6-15)14-20(28-22)29-23-27-19-7-4-12-24-21(19)31-23/h1-7,12,14,16,18,30H,8-11,13H2,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442145

(CHEMBL2441271)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(Cl)cc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;33.51,-3.74,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H24ClN5OS/c25-16-6-11-20-21(13-16)32-24(28-20)30-22-14-18(12-15-4-2-1-3-5-15)27-23(29-22)26-17-7-9-19(31)10-8-17/h1-6,11,13-14,17,19,31H,7-10,12H2,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50403876
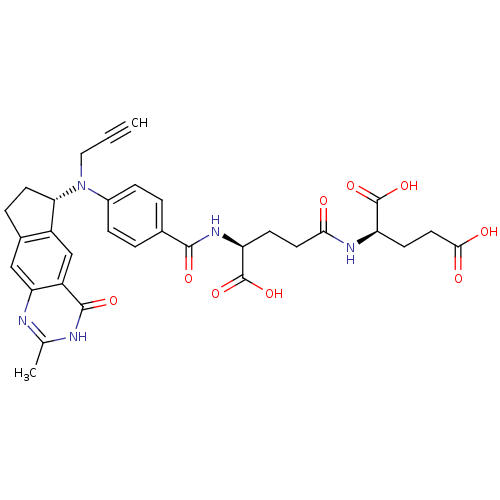
(CHEMBL323098)Show SMILES Cc1nc2cc3CC[C@H](N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C32H33N5O9/c1-3-14-37(26-11-6-19-15-25-22(16-21(19)26)30(42)34-17(2)33-25)20-7-4-18(5-8-20)29(41)36-24(32(45)46)9-12-27(38)35-23(31(43)44)10-13-28(39)40/h1,4-5,7-8,15-16,23-24,26H,6,9-14H2,2H3,(H,35,38)(H,36,41)(H,39,40)(H,43,44)(H,45,46)(H,33,34,42)/t23-,24+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120502

(2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...)Show SMILES CC(C)(C)N1N=C2CCN(CC2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccc(F)c(F)c1)NC(=O)C(C)(C)N |t:5| Show InChI InChI=1S/C31H39F2N5O4/c1-29(2,3)38-28(41)31(16-20-9-7-6-8-10-20)19-37(14-13-25(31)36-38)26(39)24(35-27(40)30(4,5)34)18-42-17-21-11-12-22(32)23(33)15-21/h6-12,15,24H,13-14,16-19,34H2,1-5H3,(H,35,40)/t24-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442146
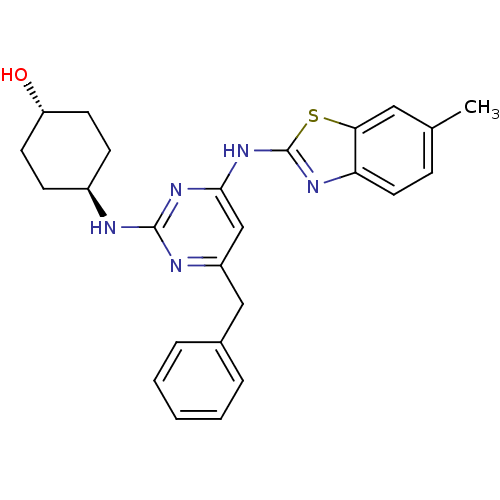
(CHEMBL2441270)Show SMILES Cc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:24.25,wD:21.21,(33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H27N5OS/c1-16-7-12-21-22(13-16)32-25(28-21)30-23-15-19(14-17-5-3-2-4-6-17)27-24(29-23)26-18-8-10-20(31)11-9-18/h2-7,12-13,15,18,20,31H,8-11,14H2,1H3,(H2,26,27,28,29,30)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50088161

(2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...)Show SMILES Cc1nc2cc3CCC(N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C32H33N5O9/c1-3-14-37(26-11-6-19-15-25-22(16-21(19)26)30(42)34-17(2)33-25)20-7-4-18(5-8-20)29(41)36-24(32(45)46)9-12-27(38)35-23(31(43)44)10-13-28(39)40/h1,4-5,7-8,15-16,23-24,26H,6,9-14H2,2H3,(H,35,38)(H,36,41)(H,39,40)(H,43,44)(H,45,46)(H,33,34,42)/t23-,24+,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120504
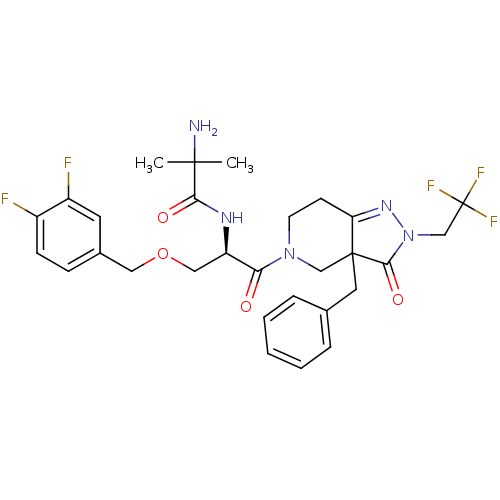
(2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccc(F)c(F)c1)C(=O)N1CCC2=NN(CC(F)(F)F)C(=O)C2(Cc2ccccc2)C1 |t:25| Show InChI InChI=1S/C29H32F5N5O4/c1-27(2,35)25(41)36-22(15-43-14-19-8-9-20(30)21(31)12-19)24(40)38-11-10-23-28(16-38,13-18-6-4-3-5-7-18)26(42)39(37-23)17-29(32,33)34/h3-9,12,22H,10-11,13-17,35H2,1-2H3,(H,36,41)/t22-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50442149

(CHEMBL2441267)Show SMILES Cc1cnc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)s1 |r,wU:22.23,wD:19.19,(57.53,-16.46,;56.01,-16.27,;55.28,-14.93,;53.76,-15.22,;53.57,-16.74,;52.23,-17.49,;52.2,-19.03,;50.85,-19.77,;50.82,-21.31,;49.48,-22.06,;48.11,-21.34,;46.81,-22.18,;45.44,-21.47,;45.37,-19.93,;46.67,-19.1,;48.04,-19.8,;52.14,-22.11,;53.49,-21.36,;54.81,-22.16,;54.78,-23.7,;53.43,-24.44,;53.4,-25.98,;54.73,-26.78,;54.7,-28.32,;56.07,-26.03,;56.1,-24.49,;53.52,-19.82,;54.97,-17.41,)| Show InChI InChI=1S/C21H25N5OS/c1-14-13-22-21(28-14)26-19-12-17(11-15-5-3-2-4-6-15)24-20(25-19)23-16-7-9-18(27)10-8-16/h2-6,12-13,16,18,27H,7-11H2,1H3,(H2,22,23,24,25,26)/t16-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442139
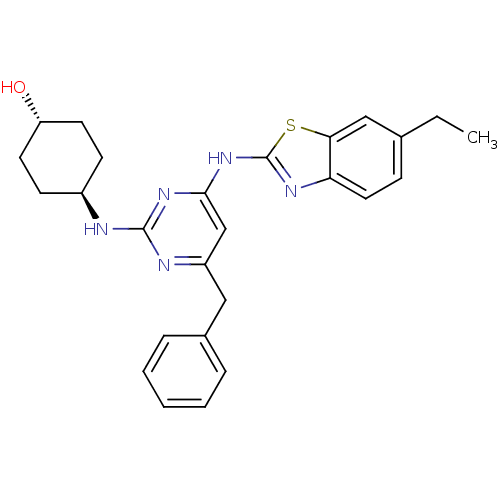
(CHEMBL2441273)Show SMILES CCc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:25.26,wD:22.22,(34.44,-4.96,;33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C26H29N5OS/c1-2-17-8-13-22-23(15-17)33-26(29-22)31-24-16-20(14-18-6-4-3-5-7-18)28-25(30-24)27-19-9-11-21(32)12-10-19/h3-8,13,15-16,19,21,32H,2,9-12,14H2,1H3,(H2,27,28,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50403877
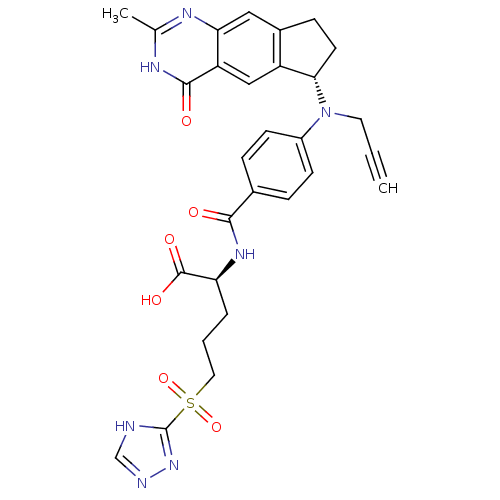
(CHEMBL103105)Show SMILES Cc1nc2cc3CC[C@H](N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCCS(=O)(=O)c4nnc[nH]4)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C29H29N7O6S/c1-3-12-36(25-11-8-19-14-24-22(15-21(19)25)27(38)33-17(2)32-24)20-9-6-18(7-10-20)26(37)34-23(28(39)40)5-4-13-43(41,42)29-30-16-31-35-29/h1,6-7,9-10,14-16,23,25H,4-5,8,11-13H2,2H3,(H,34,37)(H,39,40)(H,30,31,35)(H,32,33,38)/t23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50088164

((S)-2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cy...)Show SMILES Cc1nc2cc3CCC(N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCCS(=O)(=O)c4nnc[nH]4)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C29H29N7O6S/c1-3-12-36(25-11-8-19-14-24-22(15-21(19)25)27(38)33-17(2)32-24)20-9-6-18(7-10-20)26(37)34-23(28(39)40)5-4-13-43(41,42)29-30-16-31-35-29/h1,6-7,9-10,14-16,23,25H,4-5,8,11-13H2,2H3,(H,34,37)(H,39,40)(H,30,31,35)(H,32,33,38)/t23-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442147
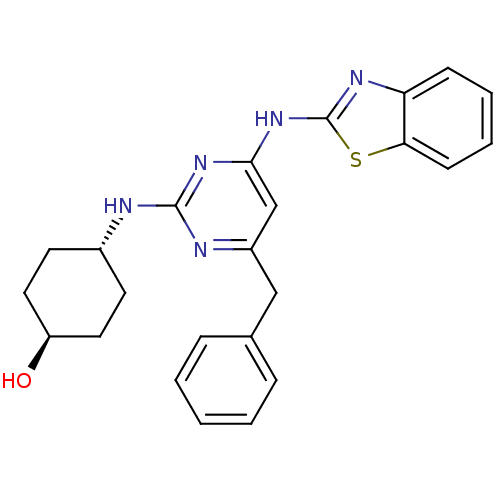
(CHEMBL2441269)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccccc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H25N5OS/c30-19-12-10-17(11-13-19)25-23-26-18(14-16-6-2-1-3-7-16)15-22(28-23)29-24-27-20-8-4-5-9-21(20)31-24/h1-9,15,17,19,30H,10-14H2,(H2,25,26,27,28,29)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50434481

(CHEMBL2385132 | CHEMBL2385133 | CHEMBL2385134)Show SMILES CCN(Cc1cccnc1)C(=O)CN(c1ccc(OC)nc1)S(=O)(=O)c1ccccc1C Show InChI InChI=1S/C23H26N4O4S/c1-4-26(16-19-9-7-13-24-14-19)23(28)17-27(20-11-12-22(31-3)25-15-20)32(29,30)21-10-6-5-8-18(21)2/h5-15H,4,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Antagonist activity at OX2 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 3389-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.079
BindingDB Entry DOI: 10.7270/Q279461B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM218837

(TBMB-PK15 (10))Show InChI InChI=1S/C12H18S3/c1-13-7-10-4-11(8-14-2)6-12(5-10)9-15-3/h4-6H,7-9H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow
| Assay Description
The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... |
Chembiochem 18: 387-395 (2017)
Article DOI: 10.1002/cbic.201600612
BindingDB Entry DOI: 10.7270/Q2H9942B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442140
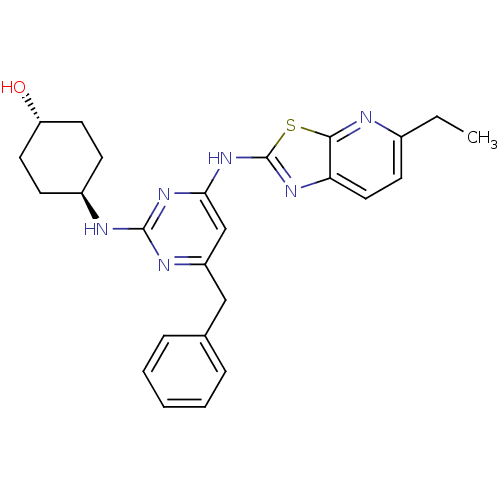
(CHEMBL2441277)Show SMILES CCc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.44,-4.96,;33.5,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H28N6OS/c1-2-17-10-13-21-23(26-17)33-25(29-21)31-22-15-19(14-16-6-4-3-5-7-16)28-24(30-22)27-18-8-11-20(32)12-9-18/h3-7,10,13,15,18,20,32H,2,8-9,11-12,14H2,1H3,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442149

(CHEMBL2441267)Show SMILES Cc1cnc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)s1 |r,wU:22.23,wD:19.19,(57.53,-16.46,;56.01,-16.27,;55.28,-14.93,;53.76,-15.22,;53.57,-16.74,;52.23,-17.49,;52.2,-19.03,;50.85,-19.77,;50.82,-21.31,;49.48,-22.06,;48.11,-21.34,;46.81,-22.18,;45.44,-21.47,;45.37,-19.93,;46.67,-19.1,;48.04,-19.8,;52.14,-22.11,;53.49,-21.36,;54.81,-22.16,;54.78,-23.7,;53.43,-24.44,;53.4,-25.98,;54.73,-26.78,;54.7,-28.32,;56.07,-26.03,;56.1,-24.49,;53.52,-19.82,;54.97,-17.41,)| Show InChI InChI=1S/C21H25N5OS/c1-14-13-22-21(28-14)26-19-12-17(11-15-5-3-2-4-6-15)24-20(25-19)23-16-7-9-18(27)10-8-16/h2-6,12-13,16,18,27H,7-11H2,1H3,(H2,22,23,24,25,26)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50403878

(CHEMBL320651)Show SMILES Cc1nc2cc3CC[C@@H](N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)c4nnn[nH]4)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C32H33N9O7/c1-3-14-41(26-11-6-19-15-25-22(16-21(19)26)31(46)34-17(2)33-25)20-7-4-18(5-8-20)30(45)36-24(32(47)48)9-12-27(42)35-23(10-13-28(43)44)29-37-39-40-38-29/h1,4-5,7-8,15-16,23-24,26H,6,9-14H2,2H3,(H,35,42)(H,36,45)(H,43,44)(H,47,48)(H,33,34,46)(H,37,38,39,40)/t23-,24+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50403880
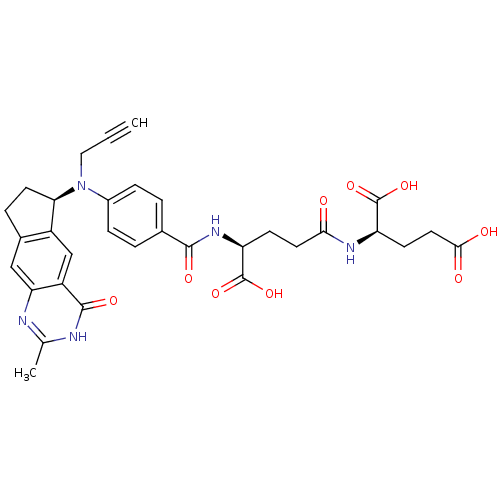
(CHEMBL317717)Show SMILES Cc1nc2cc3CC[C@@H](N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c3cc2c(=O)[nH]1 Show InChI InChI=1S/C32H33N5O9/c1-3-14-37(26-11-6-19-15-25-22(16-21(19)26)30(42)34-17(2)33-25)20-7-4-18(5-8-20)29(41)36-24(32(45)46)9-12-27(38)35-23(31(43)44)10-13-28(39)40/h1,4-5,7-8,15-16,23-24,26H,6,9-14H2,2H3,(H,35,38)(H,36,41)(H,39,40)(H,43,44)(H,45,46)(H,33,34,42)/t23-,24+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442150
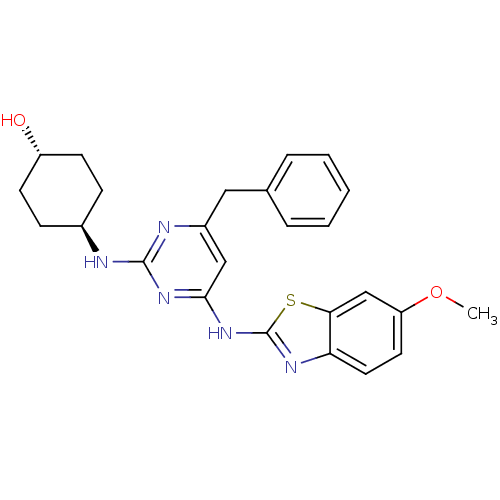
(CHEMBL2441112)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:25.26,wD:22.22,(39.46,-21.76,;38.71,-20.41,;37.17,-20.38,;36.42,-19.04,;34.89,-19.01,;34.11,-20.32,;32.6,-20.62,;32.41,-22.14,;31.06,-22.88,;31.04,-24.42,;29.69,-25.17,;29.66,-26.7,;28.32,-27.45,;26.94,-26.74,;25.65,-27.57,;24.28,-26.86,;24.21,-25.33,;25.51,-24.5,;26.87,-25.2,;30.98,-27.5,;32.32,-26.75,;33.65,-27.55,;33.62,-29.09,;32.27,-29.83,;32.24,-31.37,;33.56,-32.17,;33.53,-33.71,;34.9,-31.42,;34.93,-29.88,;32.35,-25.21,;33.8,-22.8,;34.85,-21.67,;36.38,-21.7,)| Show InChI InChI=1S/C25H27N5O2S/c1-32-20-11-12-21-22(15-20)33-25(28-21)30-23-14-18(13-16-5-3-2-4-6-16)27-24(29-23)26-17-7-9-19(31)10-8-17/h2-6,11-12,14-15,17,19,31H,7-10,13H2,1H3,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50442153
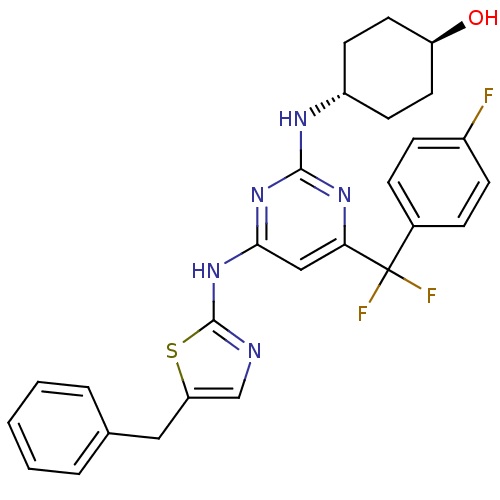
(CHEMBL2441283)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ncc(Cc3ccccc3)s2)cc(n1)C(F)(F)c1ccc(F)cc1 |r,wU:1.0,wD:4.7,(16.64,-34.58,;16.67,-33.04,;15.34,-32.24,;15.37,-30.7,;16.72,-29.96,;18.04,-30.75,;18.01,-32.29,;16.75,-28.42,;15.43,-27.62,;15.46,-26.08,;14.14,-25.29,;14.17,-23.75,;15.51,-23,;15.7,-21.49,;17.21,-21.19,;17.96,-22.54,;19.49,-22.73,;20.42,-21.5,;21.94,-21.69,;22.87,-20.46,;22.27,-19.04,;20.73,-18.86,;19.81,-20.09,;16.91,-23.66,;12.79,-26.03,;12.76,-27.57,;14.08,-28.37,;11.42,-28.31,;10.23,-29.3,;12.4,-29.5,;10.05,-27.6,;8.75,-28.44,;7.38,-27.73,;7.31,-26.19,;5.94,-25.48,;8.61,-25.36,;9.97,-26.06,)| Show InChI InChI=1S/C27H26F3N5OS/c28-19-8-6-18(7-9-19)27(29,30)23-15-24(34-25(33-23)32-20-10-12-21(36)13-11-20)35-26-31-16-22(37-26)14-17-4-2-1-3-5-17/h1-9,15-16,20-21,36H,10-14H2,(H2,31,32,33,34,35)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120505
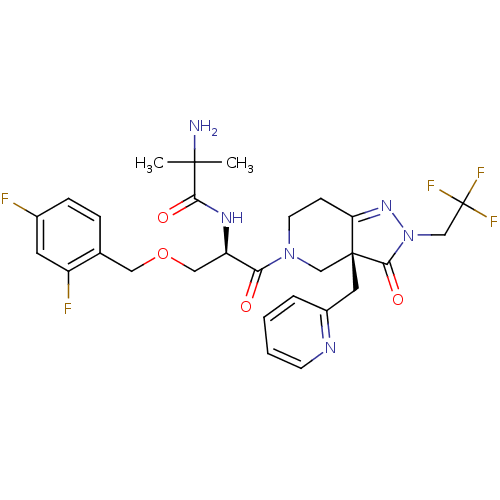
(2-Amino-N-{(R)-1-(2,4-difluoro-benzyloxymethyl)-2-...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccc(F)cc1F)C(=O)N1CCC2=NN(CC(F)(F)F)C(=O)[C@]2(Cc2ccccn2)C1 |t:25| Show InChI InChI=1S/C28H31F5N6O4/c1-26(2,34)24(41)36-21(14-43-13-17-6-7-18(29)11-20(17)30)23(40)38-10-8-22-27(15-38,12-19-5-3-4-9-35-19)25(42)39(37-22)16-28(31,32)33/h3-7,9,11,21H,8,10,12-16,34H2,1-2H3,(H,36,41)/t21-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442160
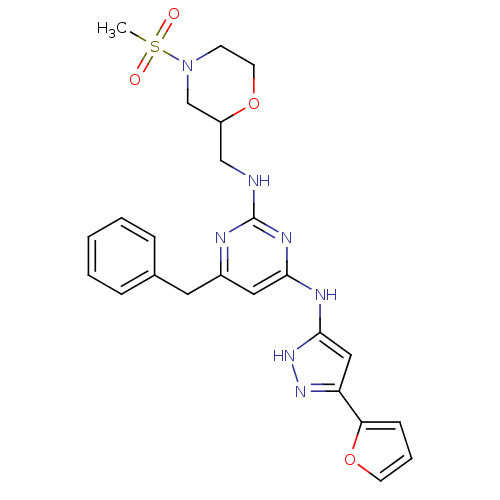
(CHEMBL2441279)Show SMILES CS(=O)(=O)N1CCOC(CNc2nc(Cc3ccccc3)cc(Nc3cc(n[nH]3)-c3ccco3)n2)C1 Show InChI InChI=1S/C24H27N7O4S/c1-36(32,33)31-9-11-34-19(16-31)15-25-24-26-18(12-17-6-3-2-4-7-17)13-22(28-24)27-23-14-20(29-30-23)21-8-5-10-35-21/h2-8,10,13-14,19H,9,11-12,15-16H2,1H3,(H3,25,26,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442155
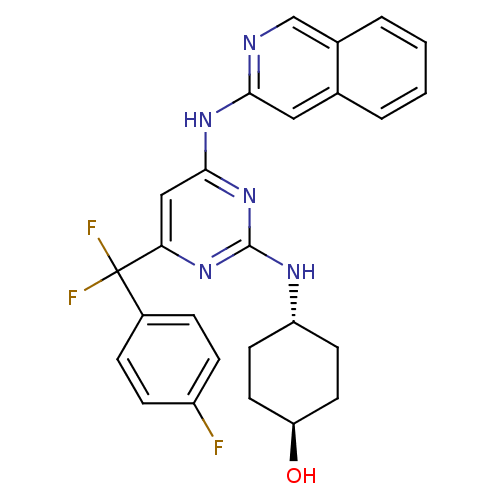
(CHEMBL2441281)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cc3ccccc3cn2)cc(n1)C(F)(F)c1ccc(F)cc1 |r,wU:1.0,wD:4.7,(43.15,-16.86,;43.18,-15.32,;41.86,-14.53,;41.89,-12.99,;43.24,-12.24,;44.55,-13.04,;44.52,-14.57,;43.27,-10.7,;41.95,-9.91,;41.97,-8.37,;40.65,-7.57,;40.68,-6.03,;41.99,-5.22,;43.35,-5.94,;44.66,-5.12,;46.01,-5.85,;47.32,-5.03,;47.26,-3.49,;45.91,-2.77,;44.61,-3.58,;43.23,-2.86,;41.93,-3.68,;39.3,-8.32,;39.28,-9.86,;40.6,-10.65,;37.93,-10.6,;36.74,-11.58,;38.91,-11.79,;36.57,-9.89,;35.27,-10.72,;33.9,-10.01,;33.83,-8.48,;32.46,-7.77,;35.13,-7.65,;36.49,-8.35,)| Show InChI InChI=1S/C26H24F3N5O/c27-19-7-5-18(6-8-19)26(28,29)22-14-24(33-23-13-16-3-1-2-4-17(16)15-30-23)34-25(32-22)31-20-9-11-21(35)12-10-20/h1-8,13-15,20-21,35H,9-12H2,(H2,30,31,32,33,34)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50083974
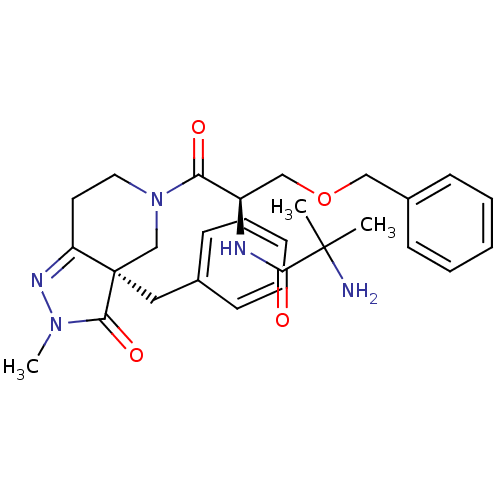
(2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...)Show SMILES CN1N=C2CCN(C[C@@]2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N |t:2| Show InChI InChI=1S/C28H35N5O4/c1-27(2,29)25(35)30-22(18-37-17-21-12-8-5-9-13-21)24(34)33-15-14-23-28(19-33,26(36)32(3)31-23)16-20-10-6-4-7-11-20/h4-13,22H,14-19,29H2,1-3H3,(H,30,35)/t22-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Mus musculus (mouse)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prostaglandin D2 from mouse CRTh2 receptor expressed in CHO cells after 2 hrs |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442144
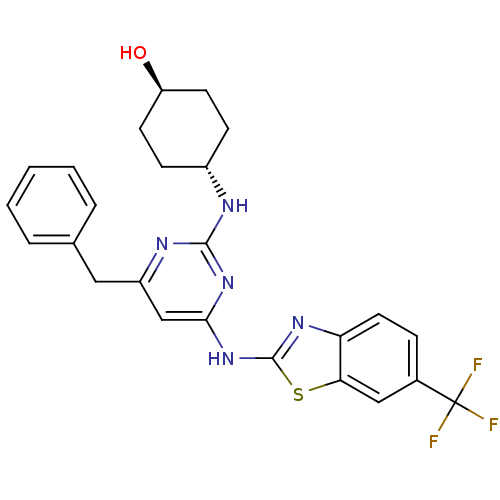
(CHEMBL2441272)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(cc3s2)C(F)(F)F)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;33.51,-3.74,;34.44,-4.96,;34.1,-2.32,;35.04,-3.73,;27.67,-9.6,)| Show InChI InChI=1S/C25H24F3N5OS/c26-25(27,28)16-6-11-20-21(13-16)35-24(31-20)33-22-14-18(12-15-4-2-1-3-5-15)30-23(32-22)29-17-7-9-19(34)10-8-17/h1-6,11,13-14,17,19,34H,7-10,12H2,(H2,29,30,31,32,33)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442161
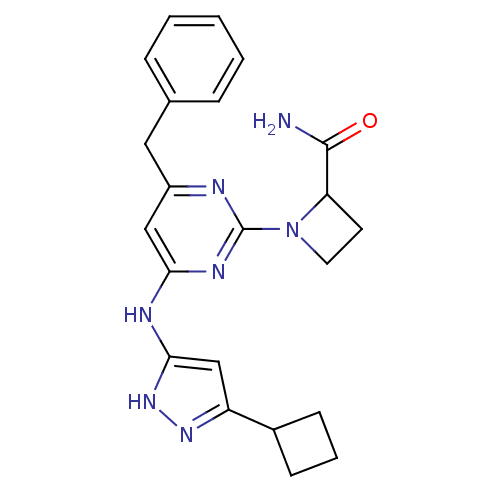
(CHEMBL2441278)Show SMILES NC(=O)C1CCN1c1nc(Cc2ccccc2)cc(Nc2cc(n[nH]2)C2CCC2)n1 Show InChI InChI=1S/C22H25N7O/c23-21(30)18-9-10-29(18)22-24-16(11-14-5-2-1-3-6-14)12-19(26-22)25-20-13-17(27-28-20)15-7-4-8-15/h1-3,5-6,12-13,15,18H,4,7-11H2,(H2,23,30)(H2,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442159
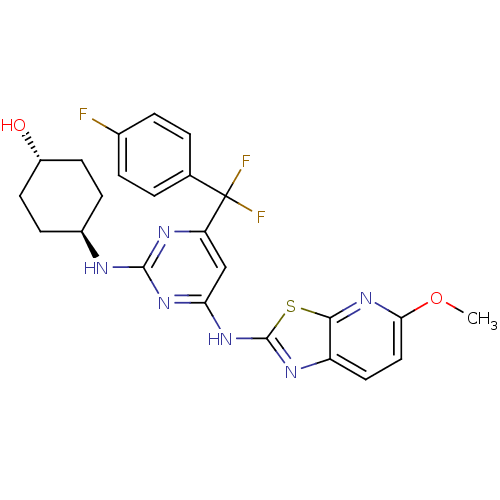
(CHEMBL2441285)Show SMILES COc1ccc2nc(Nc3cc(nc(N[C@H]4CC[C@H](O)CC4)n3)C(F)(F)c3ccc(F)cc3)sc2n1 |r,wU:18.18,wD:15.14,(18.65,-21.99,;17.9,-20.64,;16.36,-20.61,;15.62,-19.27,;14.09,-19.24,;13.3,-20.55,;11.79,-20.85,;11.6,-22.37,;10.25,-23.11,;10.23,-24.65,;8.88,-25.4,;8.85,-26.94,;10.17,-27.73,;11.52,-26.99,;12.84,-27.78,;12.81,-29.32,;11.46,-30.06,;11.43,-31.6,;12.75,-32.4,;12.73,-33.94,;14.1,-31.65,;14.13,-30.11,;11.55,-25.45,;7.51,-27.68,;6.32,-28.66,;8.28,-29.01,;6.14,-26.97,;4.84,-27.8,;3.47,-27.09,;3.4,-25.56,;2.03,-24.85,;4.7,-24.72,;6.06,-25.43,;13,-23.03,;14.04,-21.9,;15.57,-21.93,)| Show InChI InChI=1S/C24H23F3N6O2S/c1-35-20-11-10-17-21(33-20)36-23(29-17)32-19-12-18(24(26,27)13-2-4-14(25)5-3-13)30-22(31-19)28-15-6-8-16(34)9-7-15/h2-5,10-12,15-16,34H,6-9H2,1H3,(H2,28,29,30,31,32)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120503
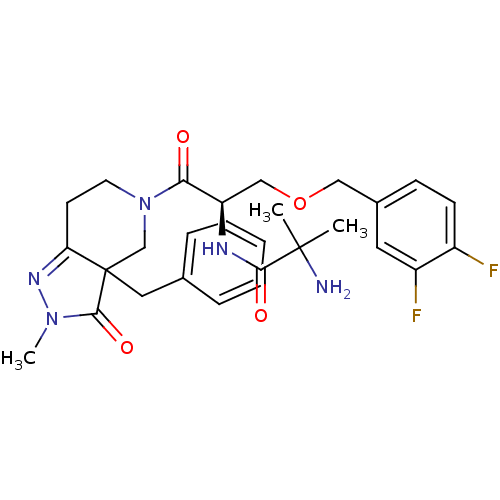
(2-Amino-N-[(R)-2-(3a-benzyl-2-methyl-3-oxo-2,3,3a,...)Show SMILES CN1N=C2CCN(CC2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccc(F)c(F)c1)NC(=O)C(C)(C)N |t:2| Show InChI InChI=1S/C28H33F2N5O4/c1-27(2,31)25(37)32-22(16-39-15-19-9-10-20(29)21(30)13-19)24(36)35-12-11-23-28(17-35,26(38)34(3)33-23)14-18-7-5-4-6-8-18/h4-10,13,22H,11-12,14-17,31H2,1-3H3,(H,32,37)/t22-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442151
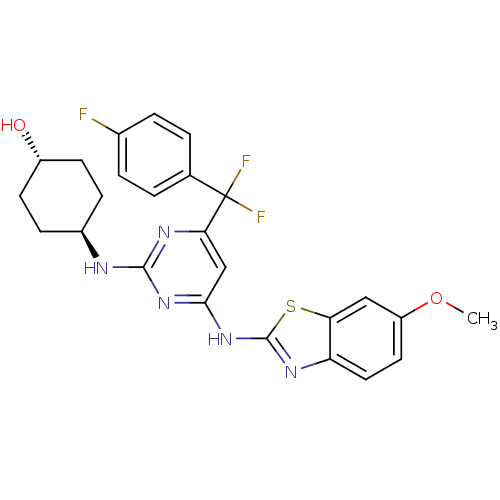
(CHEMBL2441286)Show SMILES COc1ccc2nc(Nc3cc(nc(N[C@H]4CC[C@H](O)CC4)n3)C(F)(F)c3ccc(F)cc3)sc2c1 |r,wU:18.18,wD:15.14,(40.74,-21.58,;39.99,-20.23,;38.45,-20.2,;37.7,-18.85,;36.17,-18.83,;35.39,-20.14,;33.88,-20.44,;33.69,-21.95,;32.34,-22.7,;32.31,-24.24,;30.96,-24.98,;30.93,-26.52,;32.26,-27.32,;33.61,-26.57,;34.93,-27.37,;34.9,-28.91,;33.55,-29.65,;33.52,-31.19,;34.84,-31.98,;34.81,-33.52,;36.19,-31.24,;36.21,-29.7,;33.64,-25.03,;29.59,-27.26,;28.4,-28.25,;30.69,-28.36,;28.22,-26.55,;26.93,-27.39,;25.56,-26.68,;25.49,-25.14,;24.12,-24.43,;26.79,-24.31,;28.15,-25.01,;35.08,-22.61,;36.13,-21.48,;37.66,-21.51,)| Show InChI InChI=1S/C25H24F3N5O2S/c1-35-18-10-11-19-20(12-18)36-24(30-19)33-22-13-21(25(27,28)14-2-4-15(26)5-3-14)31-23(32-22)29-16-6-8-17(34)9-7-16/h2-5,10-13,16-17,34H,6-9H2,1H3,(H2,29,30,31,32,33)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213911
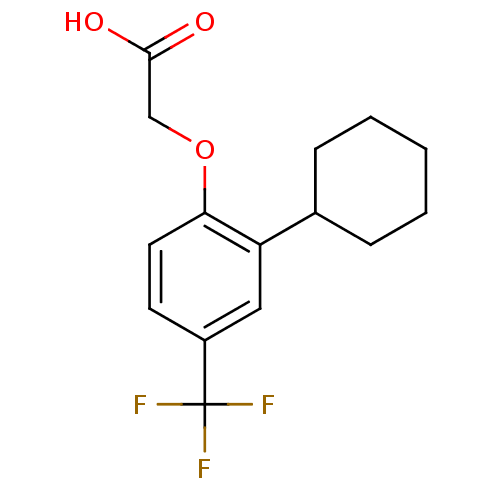
(2-(2-cyclohexyl-4-(trifluoromethyl)phenoxy)acetic ...)Show InChI InChI=1S/C15H17F3O3/c16-15(17,18)11-6-7-13(21-9-14(19)20)12(8-11)10-4-2-1-3-5-10/h6-8,10H,1-5,9H2,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thymidylate synthase of L1210 cells |
Bioorg Med Chem Lett 11: 3015-7 (2001)
BindingDB Entry DOI: 10.7270/Q2NK3G7F |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM218839
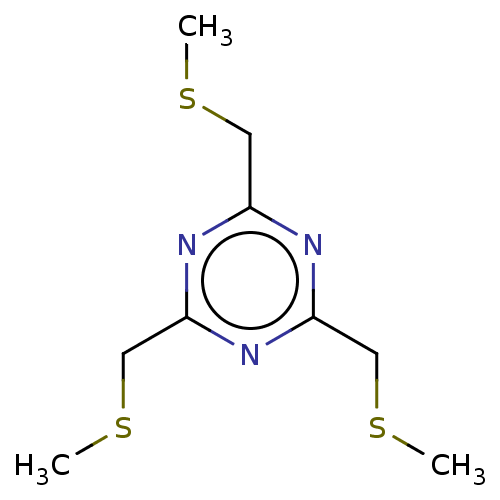
(TBMT-FX618 (16) | TBMT-PK15 (12))Show InChI InChI=1S/C9H15N3S3/c1-13-4-7-10-8(5-14-2)12-9(11-7)6-15-3/h4-6H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow
| Assay Description
The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... |
Chembiochem 18: 387-395 (2017)
Article DOI: 10.1002/cbic.201600612
BindingDB Entry DOI: 10.7270/Q2H9942B |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM218840
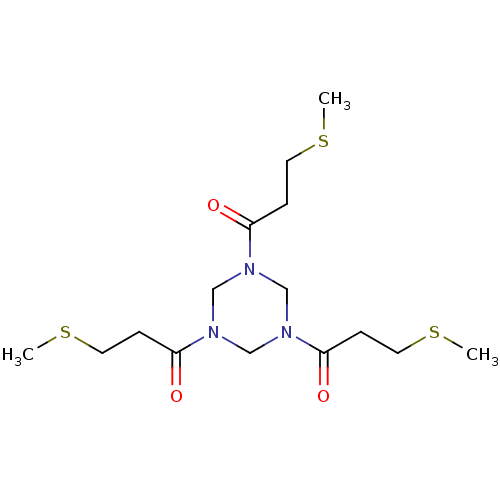
(TATA-FXII618 (14))Show InChI InChI=1S/C15H27N3O3S3/c1-22-7-4-13(19)16-10-17(14(20)5-8-23-2)12-18(11-16)15(21)6-9-24-3/h4-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Glasgow
| Assay Description
The inhibition of plasma kallikrein and coagulation factor FXII was assessed by incubating the proteases with bicyclic peptide (twofold dilutions) an... |
Chembiochem 18: 387-395 (2017)
Article DOI: 10.1002/cbic.201600612
BindingDB Entry DOI: 10.7270/Q2H9942B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Mus musculus (mouse)) | BDBM50296848
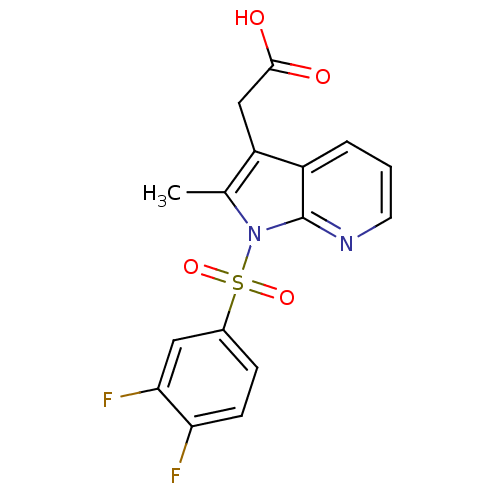
(2-(1-(3,4-difluorophenylsulfonyl)-2-methyl-1H-pyrr...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C16H12F2N2O4S/c1-9-12(8-15(21)22)11-3-2-6-19-16(11)20(9)25(23,24)10-4-5-13(17)14(18)7-10/h2-7H,8H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from mouse CRTh2 receptor expressed in K562 cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296848

(2-(1-(3,4-difluorophenylsulfonyl)-2-methyl-1H-pyrr...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C16H12F2N2O4S/c1-9-12(8-15(21)22)11-3-2-6-19-16(11)20(9)25(23,24)10-4-5-13(17)14(18)7-10/h2-7H,8H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296848

(2-(1-(3,4-difluorophenylsulfonyl)-2-methyl-1H-pyrr...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C16H12F2N2O4S/c1-9-12(8-15(21)22)11-3-2-6-19-16(11)20(9)25(23,24)10-4-5-13(17)14(18)7-10/h2-7H,8H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296849
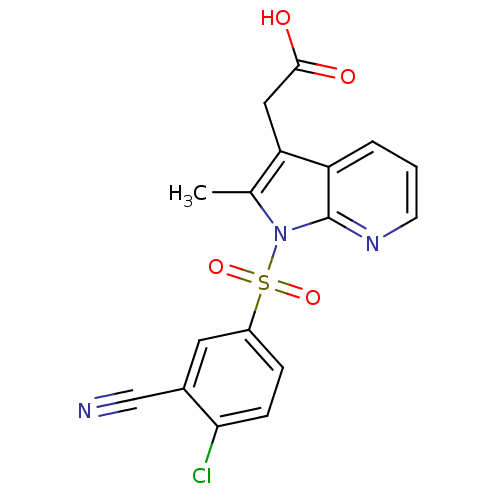
(2-(1-(4-chloro-3-cyanophenylsulfonyl)-2-methyl-1H-...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(Cl)c(c1)C#N Show InChI InChI=1S/C17H12ClN3O4S/c1-10-14(8-16(22)23)13-3-2-6-20-17(13)21(10)26(24,25)12-4-5-15(18)11(7-12)9-19/h2-7H,8H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50442299

(CHEMBL2442750)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H18N2O4S/c1-12-16(10-17(21)22)15-4-3-9-19-18(15)20(12)11-13-5-7-14(8-6-13)25(2,23)24/h3-9H,10-11H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs |
Bioorg Med Chem 21: 6582-91 (2013)
Article DOI: 10.1016/j.bmc.2013.08.025
BindingDB Entry DOI: 10.7270/Q2QR4ZKF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296850
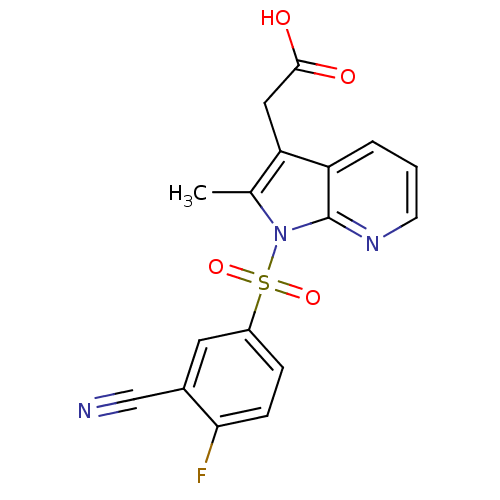
(2-(1-(3-cyano-4-fluorophenylsulfonyl)-2-methyl-1H-...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(F)c(c1)C#N Show InChI InChI=1S/C17H12FN3O4S/c1-10-14(8-16(22)23)13-3-2-6-20-17(13)21(10)26(24,25)12-4-5-15(18)11(7-12)9-19/h2-7H,8H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Calcium-dependent protein kinase 1
(Plasmodium Falciparum) | BDBM50321310

(CHEMBL4175786 | US10688093, Compound 229_0226_0284...)Show InChI InChI=1S/C15H16N4O/c1-2-8-16-14-6-7-15-17-10-13(19(15)18-14)11-4-3-5-12(20)9-11/h3-7,9-10,20H,2,8H2,1H3,(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant CDPK1 expressed in Escherichia coli expression system after 1 hr in presence of [gamma33P]ATP by scin... |
J Med Chem 61: 8061-8077 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00329
BindingDB Entry DOI: 10.7270/Q24170NC |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
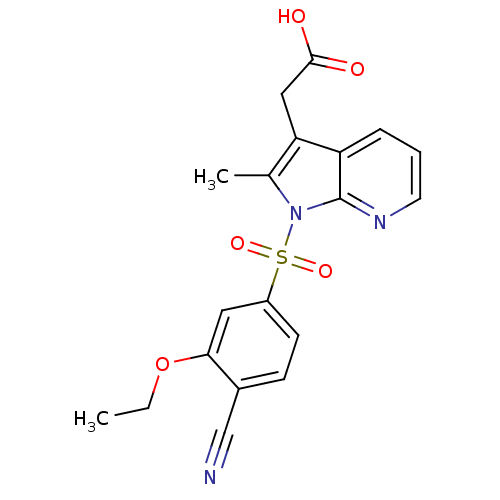
(Homo sapiens (Human)) | BDBM50296847
(2-(1-(4-cyano-3-ethoxyphenylsulfonyl)-2-methyl-1H-...)Show SMILES CCOc1cc(ccc1C#N)S(=O)(=O)n1c(C)c(CC(O)=O)c2cccnc12 Show InChI InChI=1S/C19H17N3O5S/c1-3-27-17-9-14(7-6-13(17)11-20)28(25,26)22-12(2)16(10-18(23)24)15-5-4-8-21-19(15)22/h4-9H,3,10H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
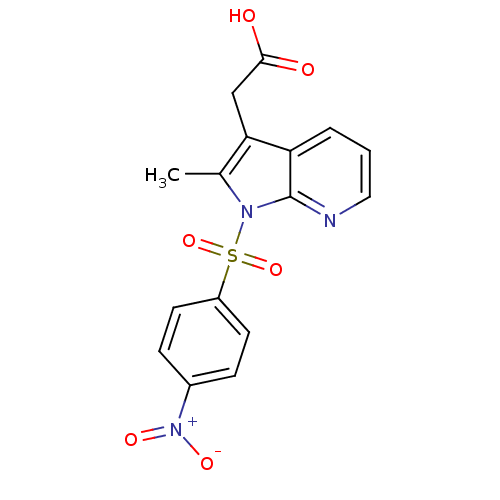
(Homo sapiens (Human)) | BDBM50296859
(2-(2-methyl-1-(4-nitrophenylsulfonyl)-1H-pyrrolo[2...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C16H13N3O6S/c1-10-14(9-15(20)21)13-3-2-8-17-16(13)18(10)26(24,25)12-6-4-11(5-7-12)19(22)23/h2-8H,9H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
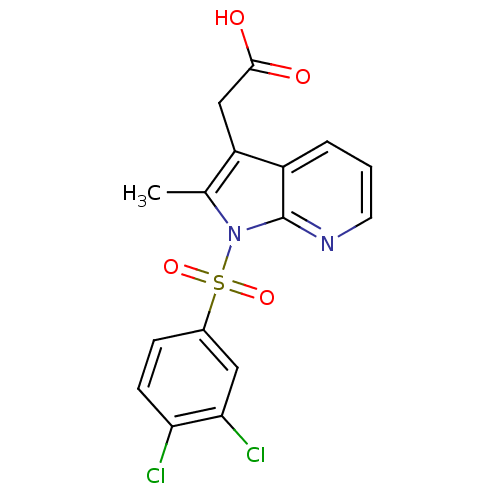
(Homo sapiens (Human)) | BDBM50296857
(2-(1-(3,4-dichlorophenylsulfonyl)-2-methyl-1H-pyrr...)Show SMILES Cc1c(CC(O)=O)c2cccnc2n1S(=O)(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C16H12Cl2N2O4S/c1-9-12(8-15(21)22)11-3-2-6-19-16(11)20(9)25(23,24)10-4-5-13(17)14(18)7-10/h2-7H,8H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296846
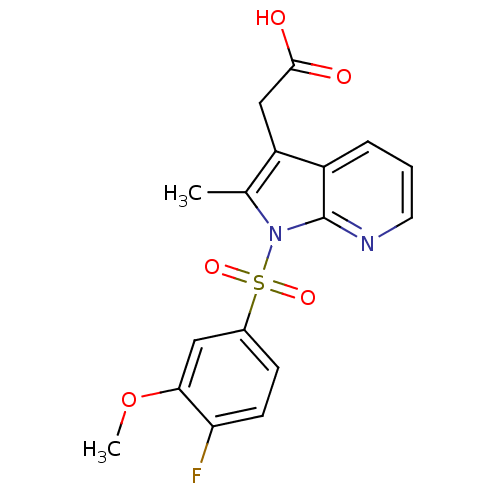
(2-(1-(4-fluoro-3-methoxyphenylsulfonyl)-2-methyl-1...)Show SMILES COc1cc(ccc1F)S(=O)(=O)n1c(C)c(CC(O)=O)c2cccnc12 Show InChI InChI=1S/C17H15FN2O5S/c1-10-13(9-16(21)22)12-4-3-7-19-17(12)20(10)26(23,24)11-5-6-14(18)15(8-11)25-2/h3-8H,9H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 4794-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.042
BindingDB Entry DOI: 10.7270/Q2BK1CCF |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213919
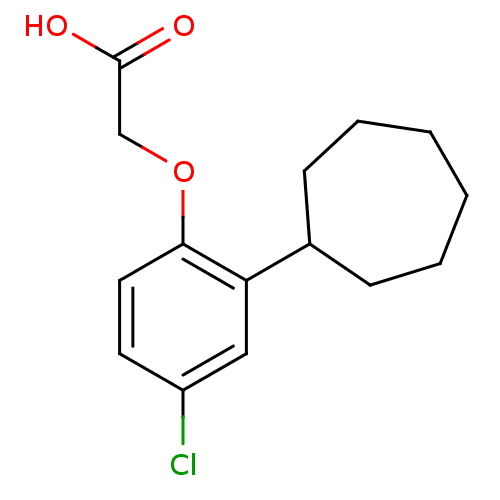
(2-(4-chloro-2-cycloheptylphenoxy)acetic acid | CHE...)Show InChI InChI=1S/C15H19ClO3/c16-12-7-8-14(19-10-15(17)18)13(9-12)11-5-3-1-2-4-6-11/h7-9,11H,1-6,10H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data