

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31801 (2-aminobenzimidazole deriv., 12) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 7 | -46.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31800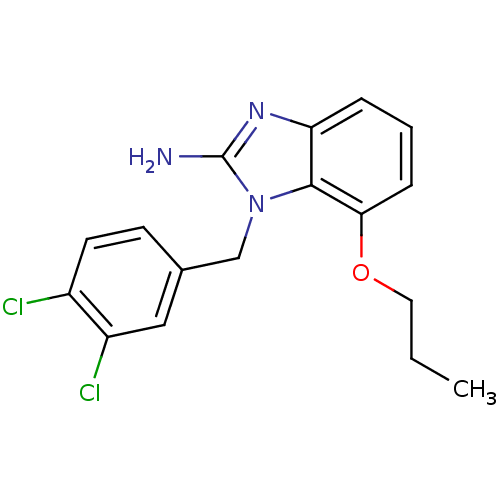 (2-aminobenzimidazole deriv., 11) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268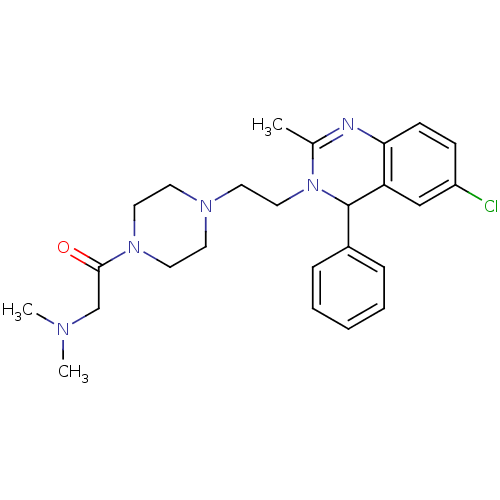 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354299 (CHEMBL1836378) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31798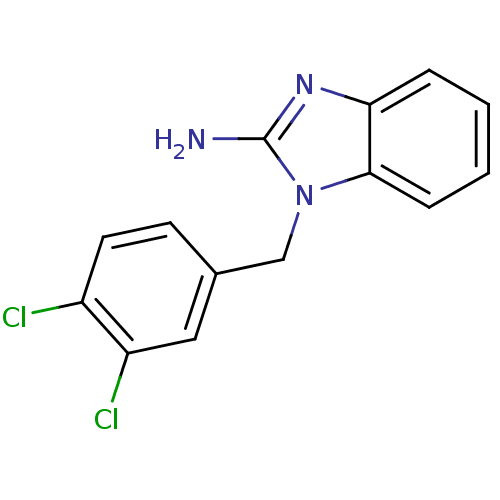 (2-aminobenzimidazole deriv., 9) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 400 | -36.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257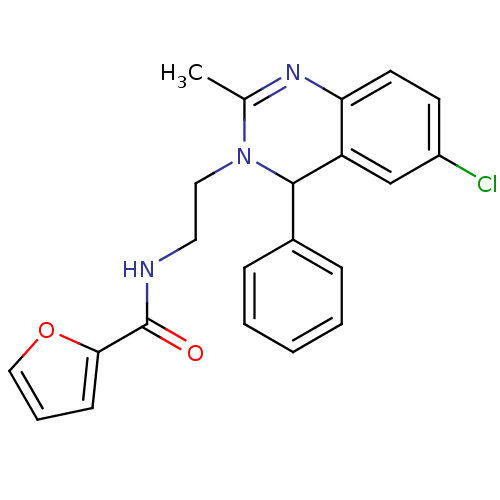 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31799 (2-aminobenzimidazole deriv., 10) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 510 | -35.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Endochitinase (Saccharomyces cerevisiae) | BDBM50331851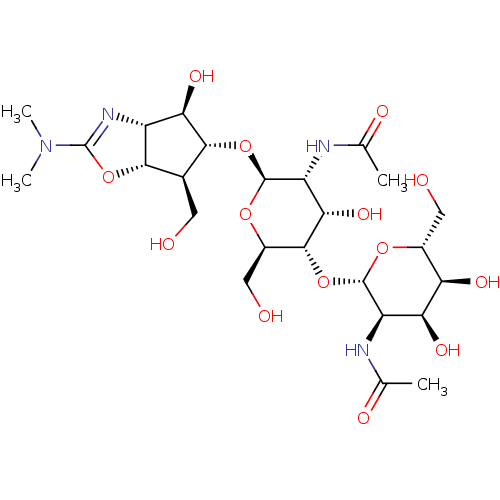 (Allosamidin | CHEMBL1230997) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae CTS1 | Bioorg Med Chem 18: 8334-40 (2010) Article DOI: 10.1016/j.bmc.2010.09.062 BindingDB Entry DOI: 10.7270/Q2NZ87WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endochitinase (Saccharomyces cerevisiae) | BDBM39302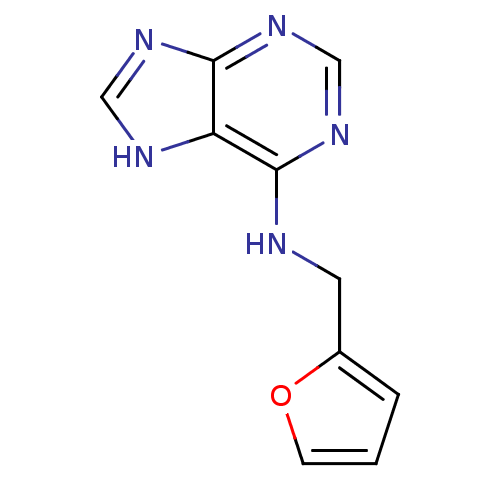 (CHEMBL228792 | MLS000101228 | N-(2-furanylmethyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae CTS1 | Bioorg Med Chem 18: 8334-40 (2010) Article DOI: 10.1016/j.bmc.2010.09.062 BindingDB Entry DOI: 10.7270/Q2NZ87WR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31795 (2-aminobenzimidazole deriv., 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | -28.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31794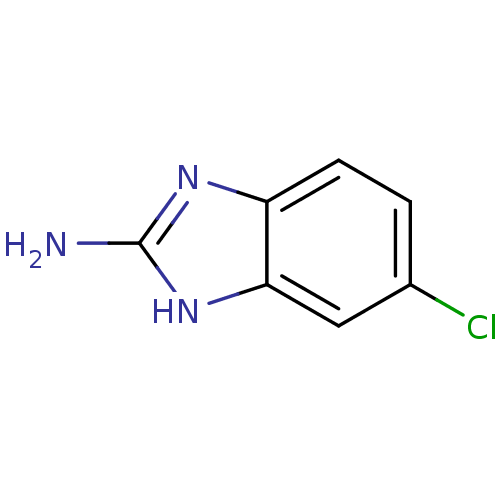 (2-aminobenzimidazole deriv., 4 | 5-Chloro-1H-benzo...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.06E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endochitinase (Saccharomyces cerevisiae) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae CTS1 | Bioorg Med Chem 18: 8334-40 (2010) Article DOI: 10.1016/j.bmc.2010.09.062 BindingDB Entry DOI: 10.7270/Q2NZ87WR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31791 (2-aminobenzothiazole deriv., 2) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.11E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31797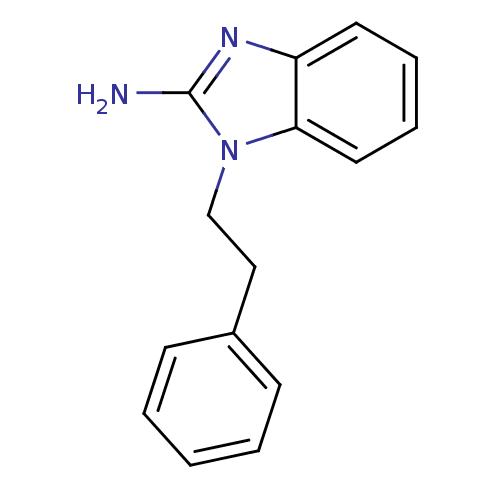 (2-aminobenzimidazole deriv., 8) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.39E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31793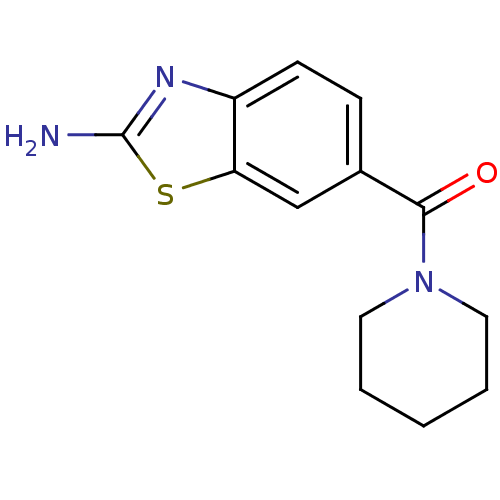 (2-aminobenzothiazole deriv., 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.41E+5 | -21.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31796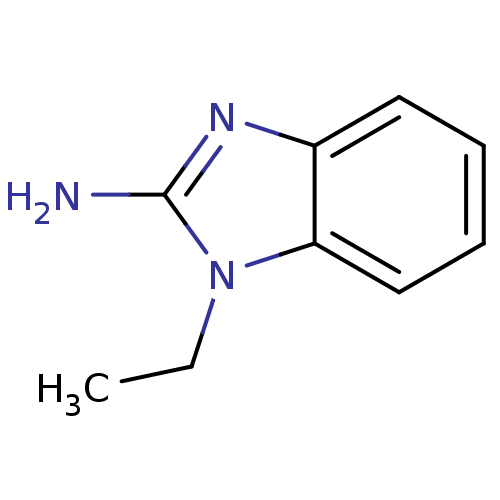 (2-aminobenzimidazole deriv., 7) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | >-21.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM7960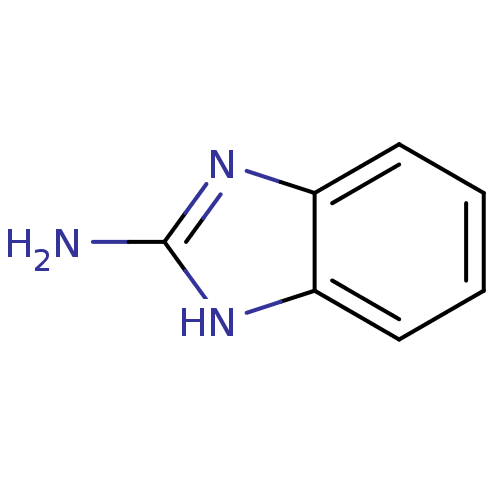 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 2.88E+5 | -20.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endochitinase (Saccharomyces cerevisiae) | BDBM50331852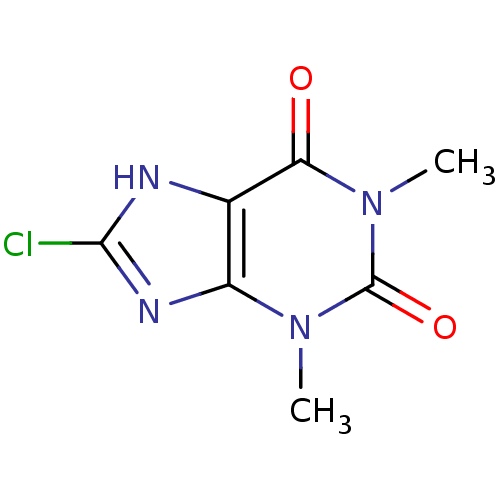 (8-CHLORO-1,3-DIMETHYL-3,7-DIHYDRO-1H-PURINE-2,6-DI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae CTS1 | Bioorg Med Chem 18: 8334-40 (2010) Article DOI: 10.1016/j.bmc.2010.09.062 BindingDB Entry DOI: 10.7270/Q2NZ87WR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase (Leishmania major) | BDBM50364113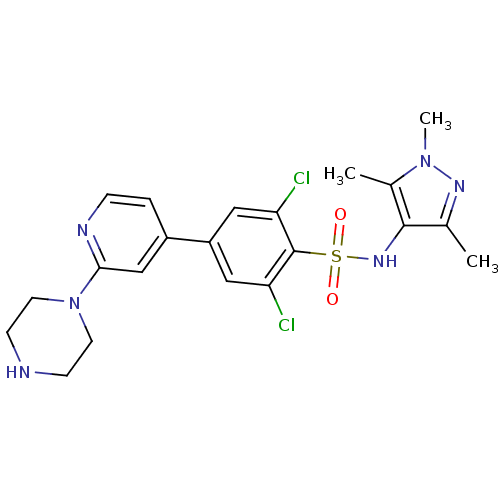 (CHEMBL1230468) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of Leishmania major N-myristoyltransferase using [3H]myristoyl-CoA and GCGGSKVKPQPPQAK(biotin)-amide as substrate preincubated for 5 mins ... | J Med Chem 55: 140-52 (2012) Article DOI: 10.1021/jm201091t BindingDB Entry DOI: 10.7270/Q25Q4WK9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364113 (CHEMBL1230468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 2 (Homo sapiens (Human)) | BDBM50364113 (CHEMBL1230468) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 2 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364113 (CHEMBL1230468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 using [3H]myristoyl-CoA and GCGGSKVKPQPPQAK(biotin)-amide as substrate preincubated for 5 mins prior sub... | J Med Chem 55: 140-52 (2012) Article DOI: 10.1021/jm201091t BindingDB Entry DOI: 10.7270/Q25Q4WK9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033418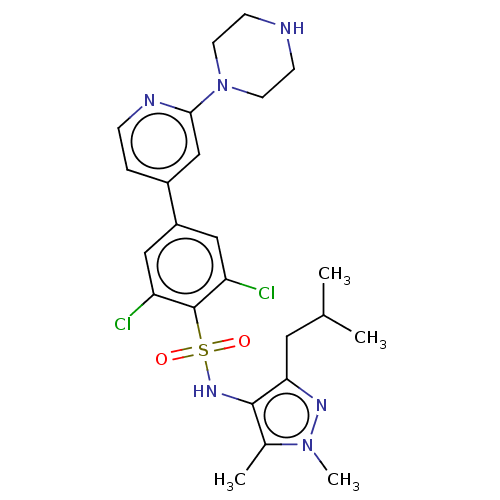 (CHEMBL3357685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364113 (CHEMBL1230468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation/luminescence counting method | J Med Chem 60: 9790-9806 (2017) Article DOI: 10.1021/acs.jmedchem.7b01255 BindingDB Entry DOI: 10.7270/Q25141NZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033616 (CHEMBL3358119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033485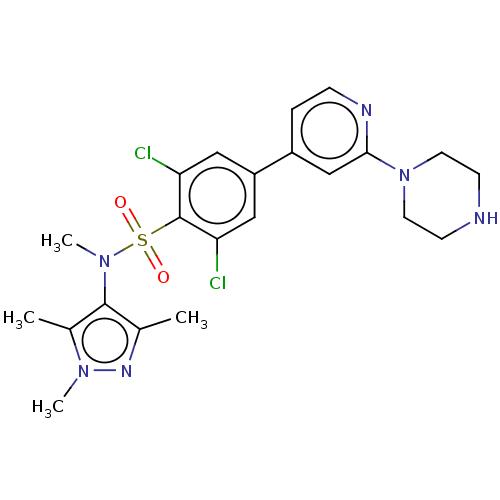 (CHEMBL3357702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033480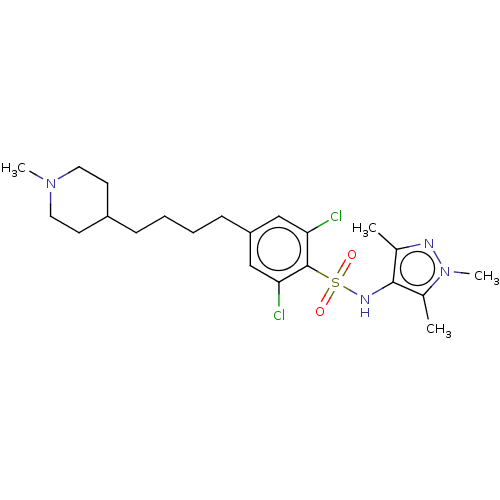 (CHEMBL3357697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033617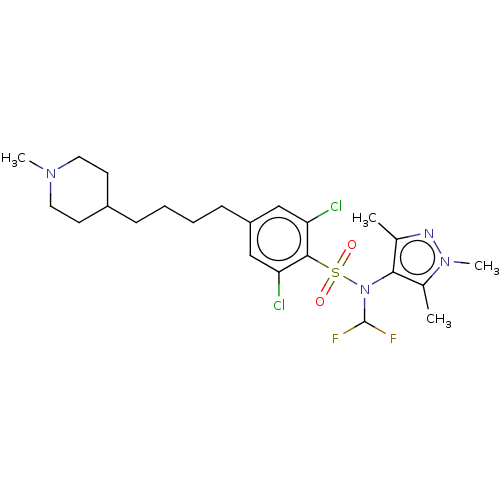 (CHEMBL3358120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation/luminescence counting method | J Med Chem 60: 9790-9806 (2017) Article DOI: 10.1021/acs.jmedchem.7b01255 BindingDB Entry DOI: 10.7270/Q25141NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025653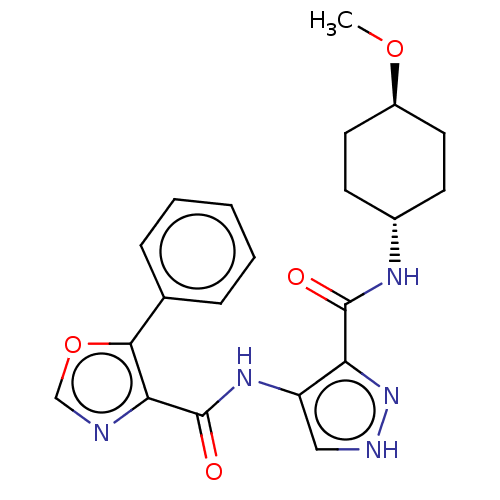 (CHEMBL3335154) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033617 (CHEMBL3358120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033618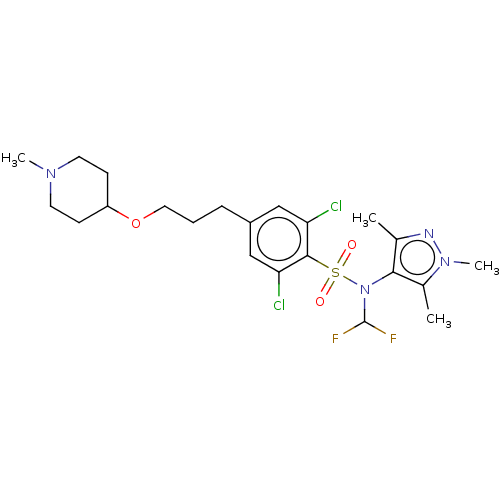 (CHEMBL3358121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025628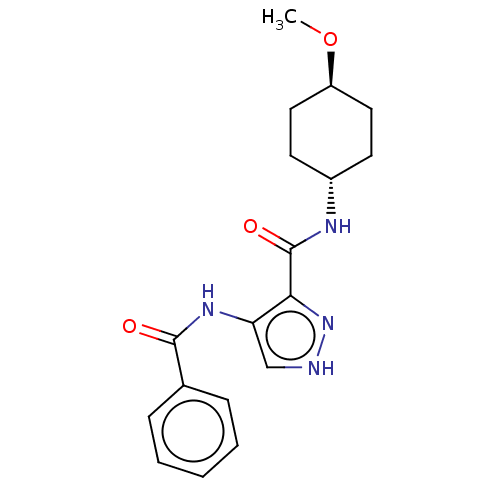 (CHEMBL3335124) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025634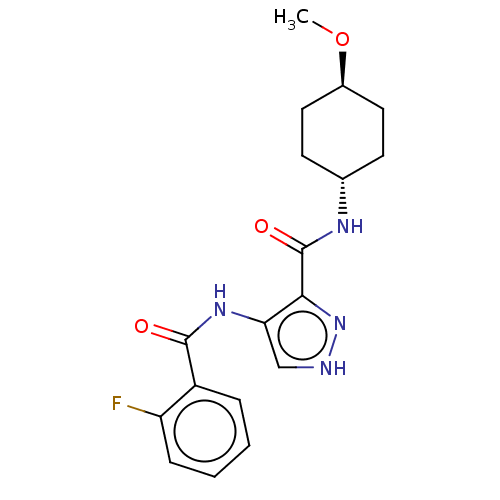 (CHEMBL3335125) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025640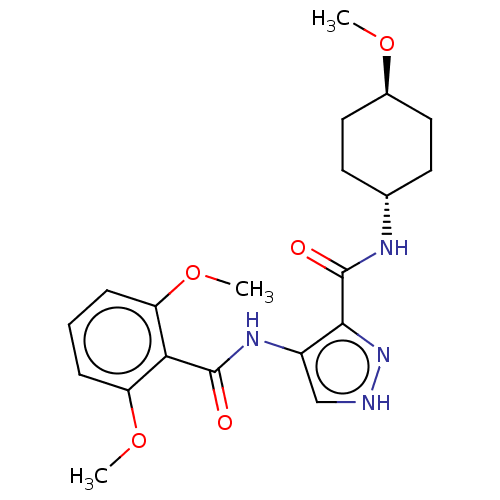 (CHEMBL3335141) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025642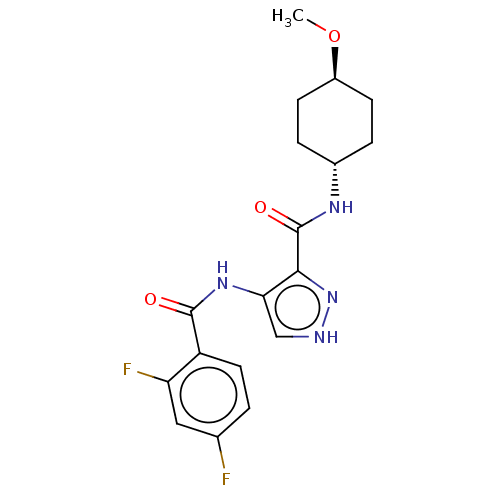 (CHEMBL3335143) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025643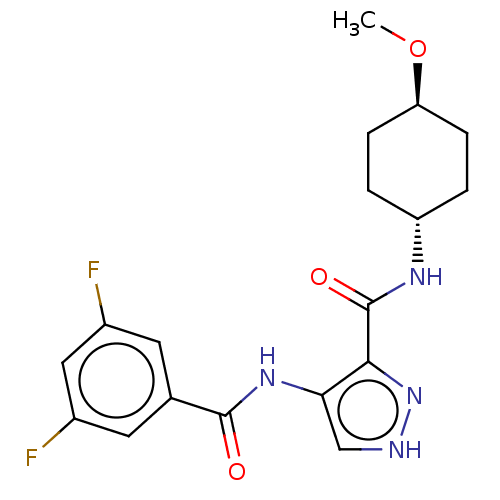 (CHEMBL3335144) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025644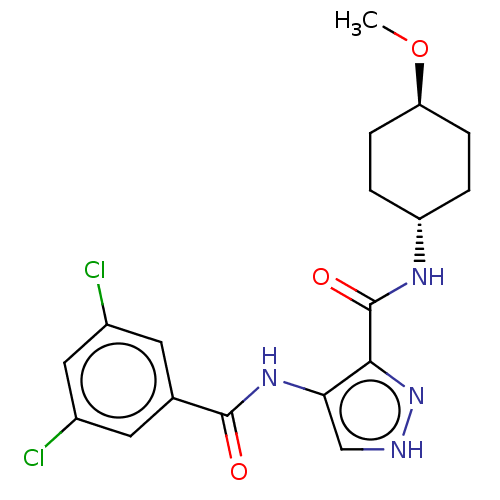 (CHEMBL3335145) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025645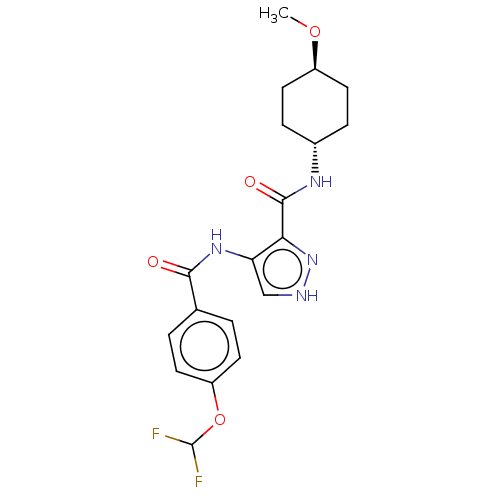 (CHEMBL3335146) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025646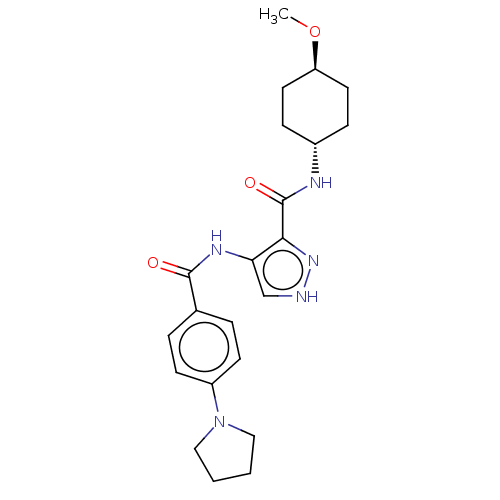 (CHEMBL3335147) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025647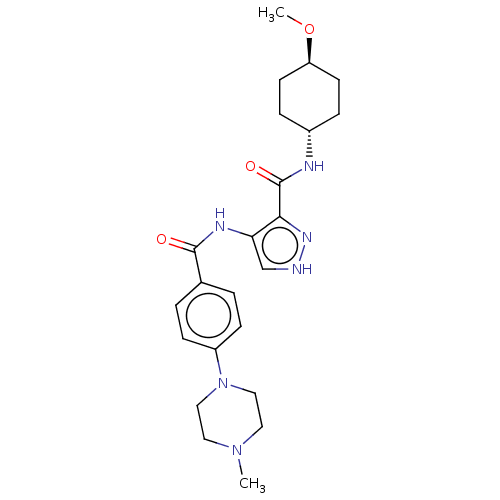 (CHEMBL3335148) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025648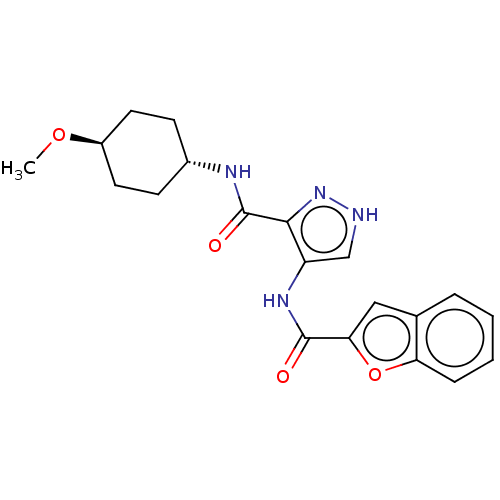 (CHEMBL3335149) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025649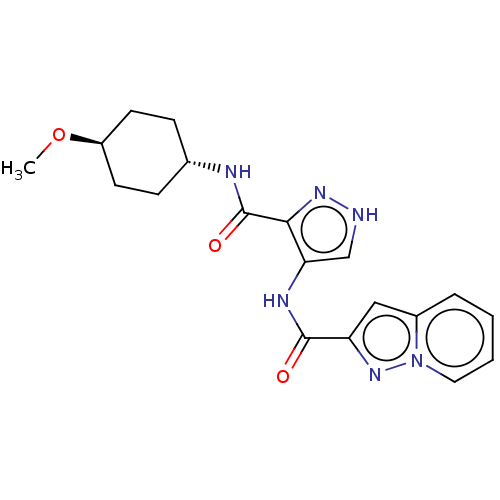 (CHEMBL3335150) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025650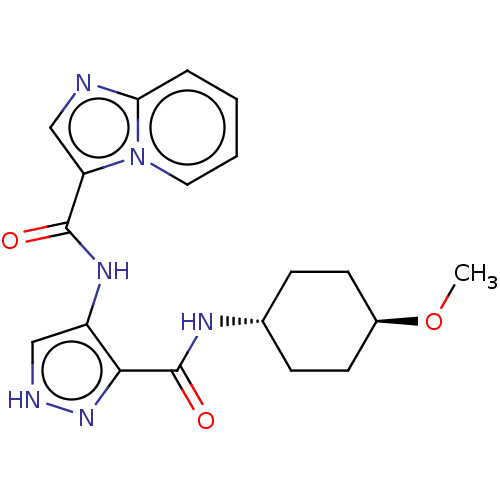 (CHEMBL3335151) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 (Homo sapiens (Human)) | BDBM50025652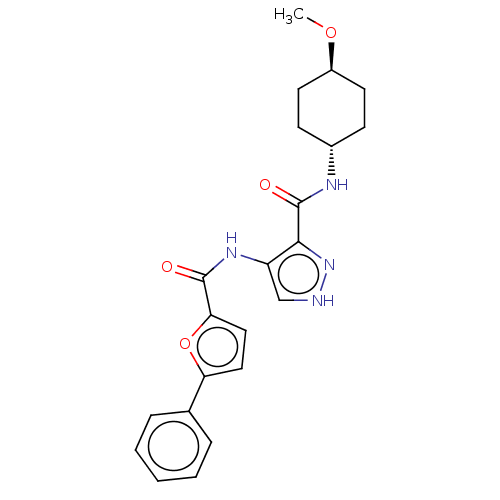 (CHEMBL3335153) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GSK3 using biotinylated GSP2 substrate by flashplate assay | J Med Chem 57: 7536-49 (2014) Article DOI: 10.1021/jm500239b BindingDB Entry DOI: 10.7270/Q2M04712 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033482 (CHEMBL3357699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364135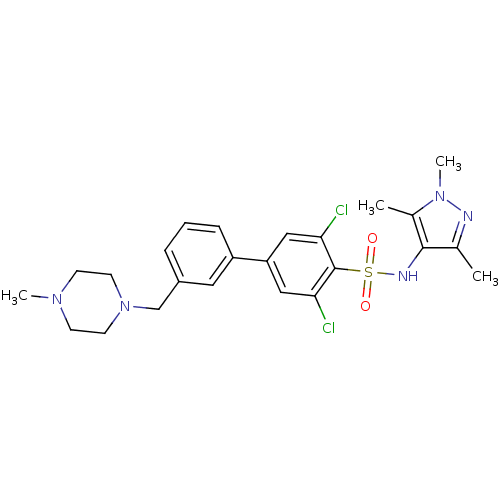 (CHEMBL1951295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 using [3H]myristoyl-CoA and GCGGSKVKPQPPQAK(biotin)-amide as substrate preincubated for 5 mins prior sub... | J Med Chem 55: 140-52 (2012) Article DOI: 10.1021/jm201091t BindingDB Entry DOI: 10.7270/Q25Q4WK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 377 total ) | Next | Last >> |