

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50078539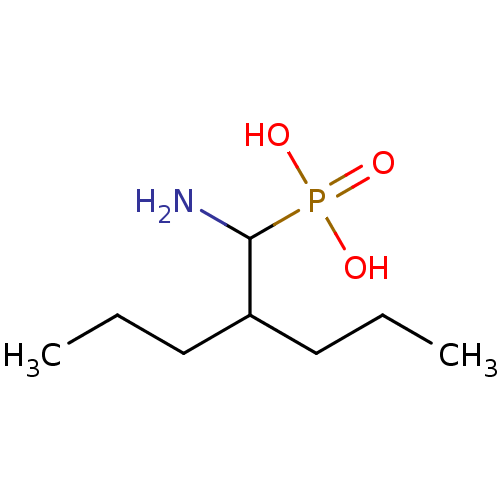 (CHEMBL3414950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Human MetAP1b using Met-pNA as substrate | J Med Chem 58: 2350-7 (2015) Article DOI: 10.1021/jm501790e BindingDB Entry DOI: 10.7270/Q2C53NJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50337147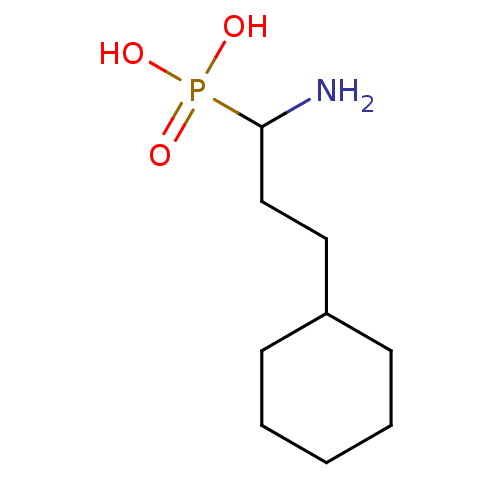 ((RS)-1-amino-3-cyclohexylpropylphosphonic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Human MetAP1b using Met-pNA as substrate | J Med Chem 58: 2350-7 (2015) Article DOI: 10.1021/jm501790e BindingDB Entry DOI: 10.7270/Q2C53NJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50078540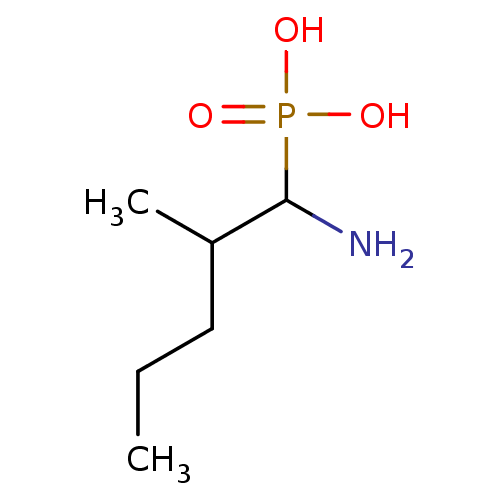 (CHEMBL1673077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Human MetAP1b using Met-pNA as substrate | J Med Chem 58: 2350-7 (2015) Article DOI: 10.1021/jm501790e BindingDB Entry DOI: 10.7270/Q2C53NJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50316031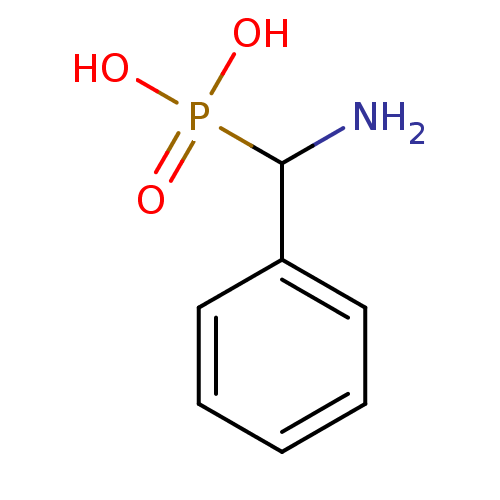 (CHEMBL1090366 | amino(phenyl)methylphosphonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Human MetAP1b using Met-pNA as substrate | J Med Chem 58: 2350-7 (2015) Article DOI: 10.1021/jm501790e BindingDB Entry DOI: 10.7270/Q2C53NJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50078541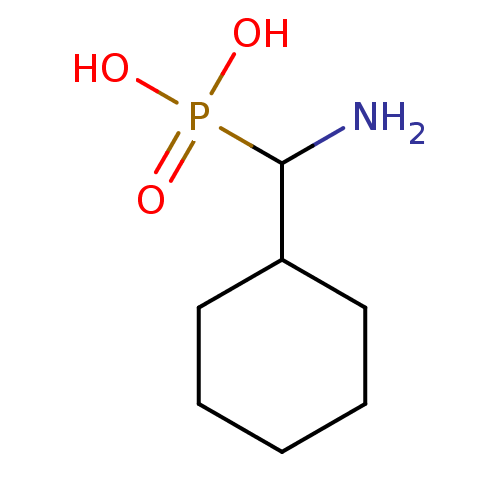 (CHEMBL3414949) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Human MetAP1b using Met-pNA as substrate | J Med Chem 58: 2350-7 (2015) Article DOI: 10.1021/jm501790e BindingDB Entry DOI: 10.7270/Q2C53NJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50337150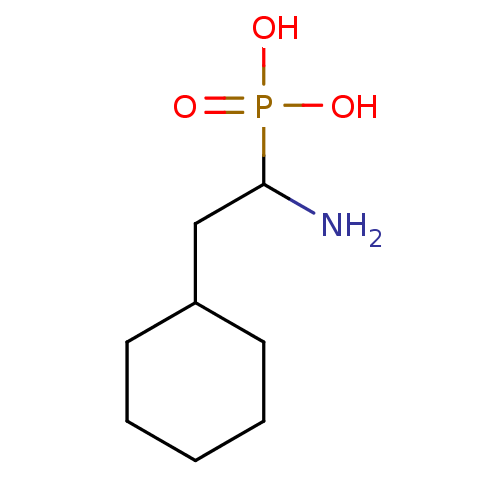 ((RS)-1-amino-2-cyclohexylethylphosphonic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of Human MetAP1b using Met-pNA as substrate | J Med Chem 58: 2350-7 (2015) Article DOI: 10.1021/jm501790e BindingDB Entry DOI: 10.7270/Q2C53NJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17847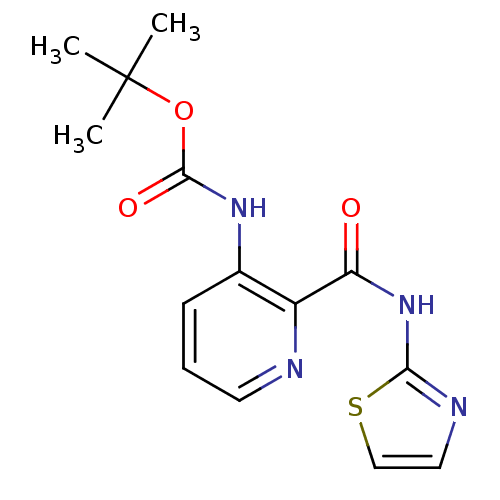 (CHEMBL327579 | pyridine-2-carboxylic acid inhibito...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50014846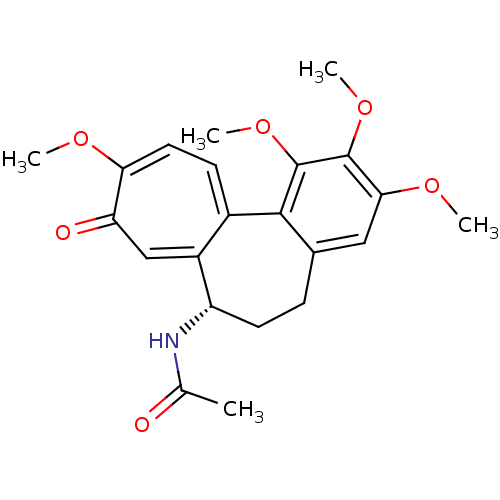 ((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin polymerization measured for 1 hr by fluorescence assay | Eur J Med Chem 90: 603-19 (2015) Article DOI: 10.1016/j.ejmech.2014.11.063 BindingDB Entry DOI: 10.7270/Q2ZG6TXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499710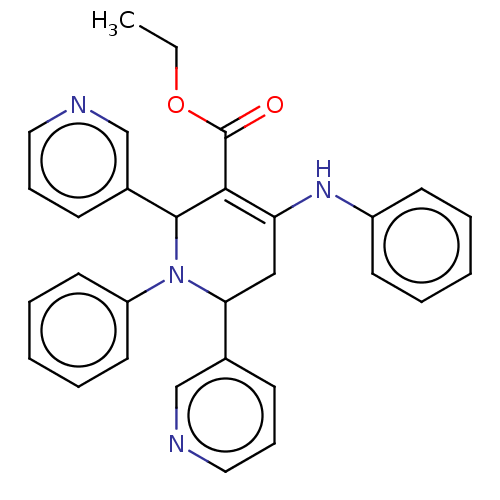 (CHEMBL516021) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277158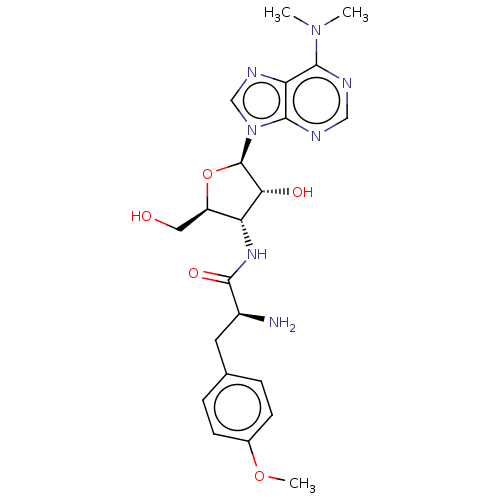 (3123L | CHEBI:17939 | CL-13900 | GNF-Pf-2016 | P-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17849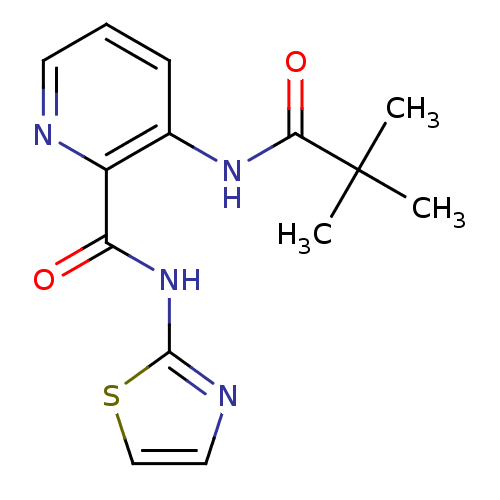 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17850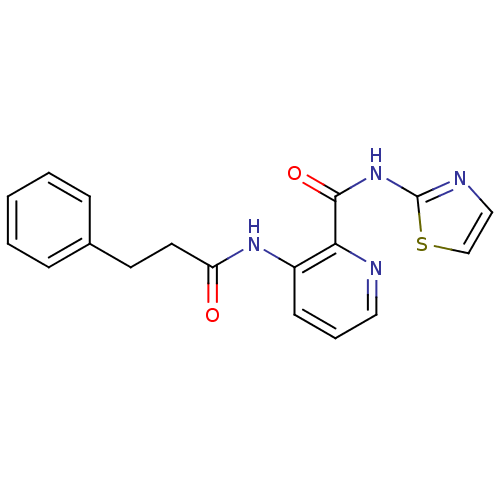 (3-(3-phenylpropanamido)-N-(1,3-thiazol-2-yl)pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17851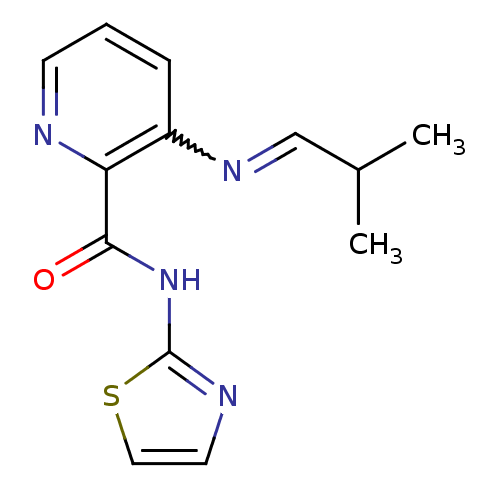 (3-[(E)-(2-methylpropylidene)amino]-N-(1,3-thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50062530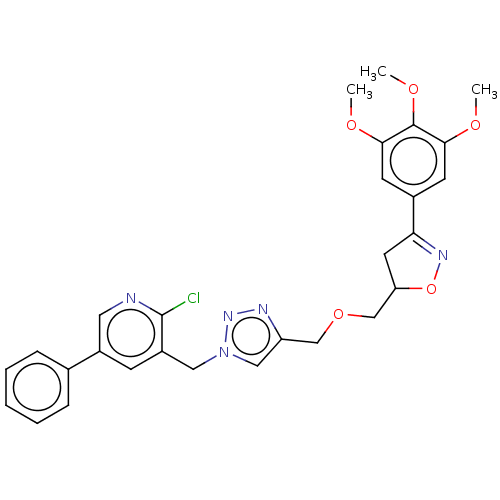 (CHEMBL3397584) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin polymerization measured for 1 hr by fluorescence assay | Eur J Med Chem 90: 603-19 (2015) Article DOI: 10.1016/j.ejmech.2014.11.063 BindingDB Entry DOI: 10.7270/Q2ZG6TXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499702 (CHEMBL3740370) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain (Bos taurus) | BDBM50062531 (CHEMBL3397585) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of bovine brain tubulin polymerization measured for 1 hr by fluorescence assay | Eur J Med Chem 90: 603-19 (2015) Article DOI: 10.1016/j.ejmech.2014.11.063 BindingDB Entry DOI: 10.7270/Q2ZG6TXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499709 (CHEMBL3741560) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499705 (CHEMBL3741724) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499712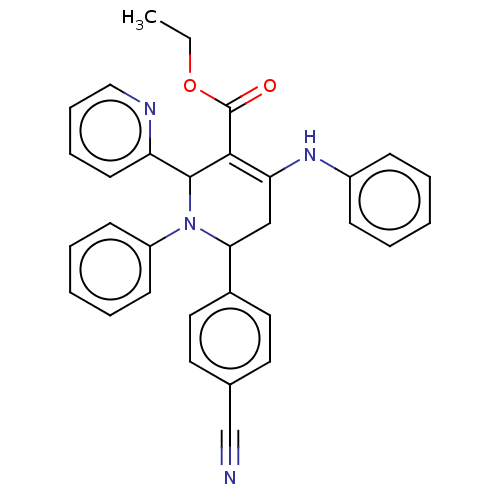 (CHEMBL3741833) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499707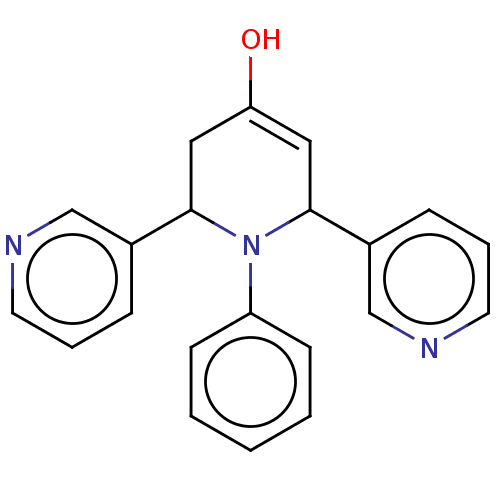 (CHEMBL3741904) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499708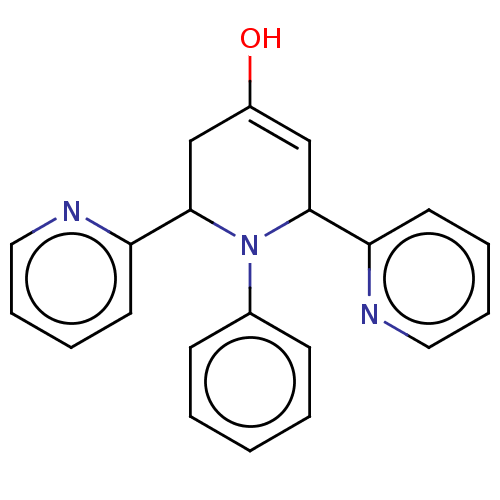 (CHEMBL3739649) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499706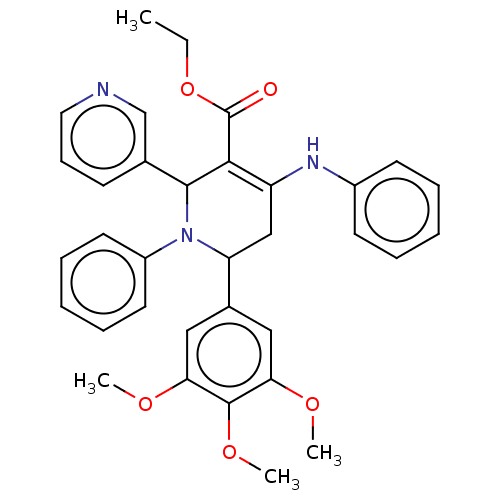 (CHEMBL3740199) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499704 (CHEMBL3740158) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499713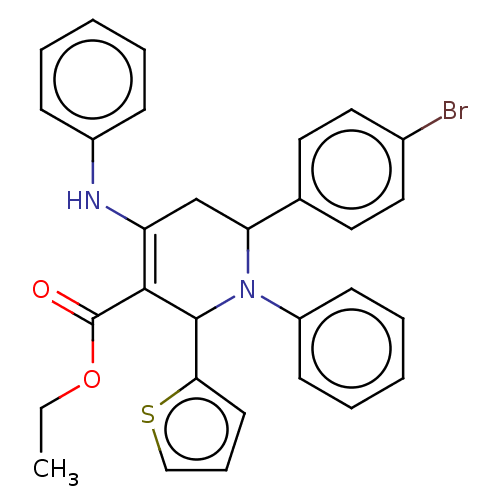 (CHEMBL3741344) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499701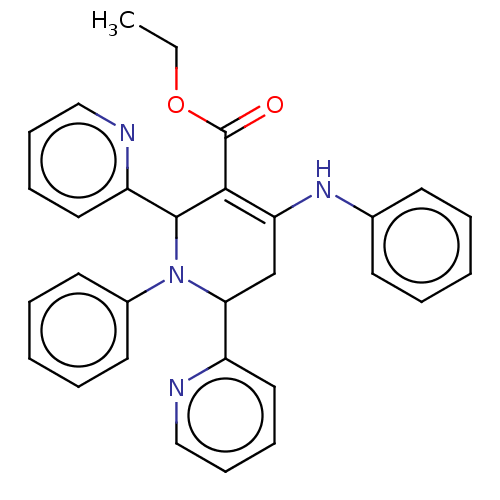 (CHEMBL3741701) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499703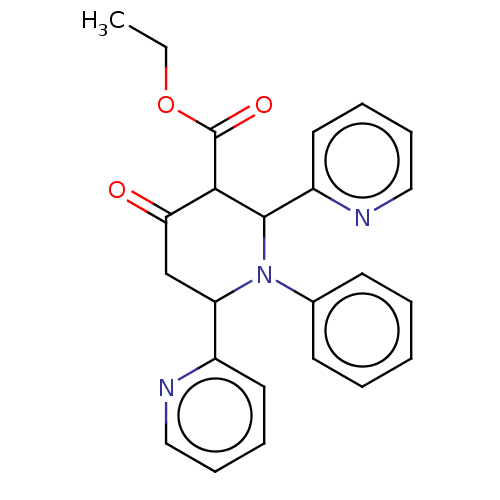 (CHEMBL3740862) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499711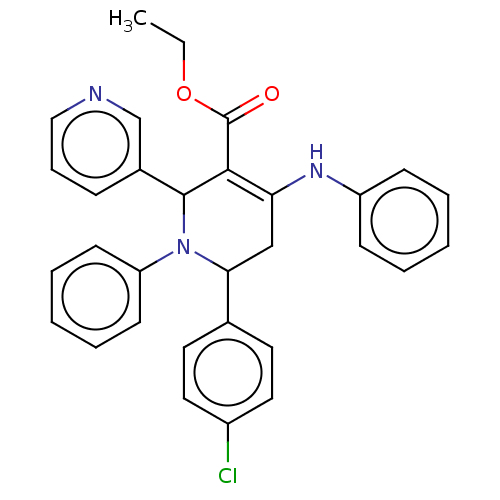 (CHEMBL3741419) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499709 (CHEMBL3741560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50277158 (3123L | CHEBI:17939 | CL-13900 | GNF-Pf-2016 | P-6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499706 (CHEMBL3740199) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499705 (CHEMBL3741724) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499704 (CHEMBL3740158) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499708 (CHEMBL3739649) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499707 (CHEMBL3741904) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499703 (CHEMBL3740862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499702 (CHEMBL3740370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499701 (CHEMBL3741701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499710 (CHEMBL516021) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499713 (CHEMBL3741344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499712 (CHEMBL3741833) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50499711 (CHEMBL3741419) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of porcine aminopeptidase-N using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17847 (CHEMBL327579 | pyridine-2-carboxylic acid inhibito...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17850 (3-(3-phenylpropanamido)-N-(1,3-thiazol-2-yl)pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17852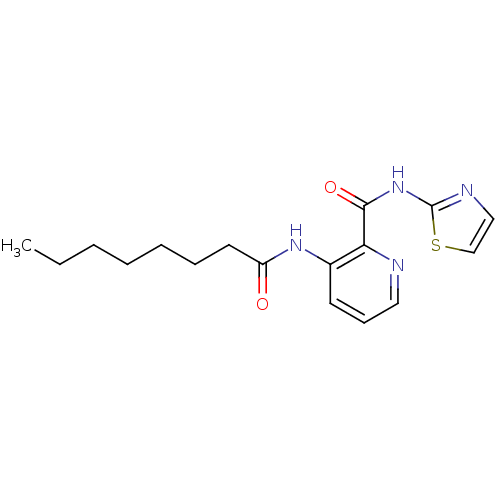 (3-octanamido-N-(1,3-thiazol-2-yl)pyridine-2-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17852 (3-octanamido-N-(1,3-thiazol-2-yl)pyridine-2-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17849 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17851 (3-[(E)-(2-methylpropylidene)amino]-N-(1,3-thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50507632 (CHEMBL4448381) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TM Bhagalpur University Curated by ChEMBL | Assay Description Anti-biofilm activity against Enterococcus faecalis after 24 hrs by XTT assay | Eur J Med Chem 163: 67-82 (2019) Article DOI: 10.1016/j.ejmech.2018.11.053 BindingDB Entry DOI: 10.7270/Q21J9F24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||