 Found 2984 hits with Last Name = 'buschauer' and Initial = 'a'
Found 2984 hits with Last Name = 'buschauer' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R (unknown origin) |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246648

((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...)Show SMILES CCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:8.8| Show InChI InChI=1S/C30H36N6O4/c1-2-32-30(40)36-29(31)33-19-9-14-25(27(38)34-20-21-15-17-24(37)18-16-21)35-28(39)26(22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-8,10-13,15-18,25-26,37H,2,9,14,19-20H2,1H3,(H,34,38)(H,35,39)(H4,31,32,33,36,40)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R (unknown origin) |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500151

(CHEMBL3746386)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCC(=O)NCCNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H41N7O5.2C2HF3O2/c1-2-28(42)35-20-21-37-33(45)40-32(34)36-19-9-14-27(30(43)38-22-23-15-17-26(41)18-16-23)39-31(44)29(24-10-5-3-6-11-24)25-12-7-4-8-13-25;2*3-2(4,5)1(6)7/h3-8,10-13,15-18,27,29,41H,2,9,14,19-22H2,1H3,(H,35,42)(H,38,43)(H,39,44)(H4,34,36,37,40,45);2*(H,6,7)/t27-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246648

((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...)Show SMILES CCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:8.8| Show InChI InChI=1S/C30H36N6O4/c1-2-32-30(40)36-29(31)33-19-9-14-25(27(38)34-20-21-15-17-24(37)18-16-21)35-28(39)26(22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-8,10-13,15-18,25-26,37H,2,9,14,19-20H2,1H3,(H,34,38)(H,35,39)(H4,31,32,33,36,40)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells |
J Med Chem 51: 8168-72 (2008)
Article DOI: 10.1021/jm801018u
BindingDB Entry DOI: 10.7270/Q2WM1D8T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500151

(CHEMBL3746386)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCC(=O)NCCNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H41N7O5.2C2HF3O2/c1-2-28(42)35-20-21-37-33(45)40-32(34)36-19-9-14-27(30(43)38-22-23-15-17-26(41)18-16-23)39-31(44)29(24-10-5-3-6-11-24)25-12-7-4-8-13-25;2*3-2(4,5)1(6)7/h3-8,10-13,15-18,27,29,41H,2,9,14,19-22H2,1H3,(H,35,42)(H,38,43)(H,39,44)(H4,34,36,37,40,45);2*(H,6,7)/t27-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells |
J Med Chem 51: 8168-72 (2008)
Article DOI: 10.1021/jm801018u
BindingDB Entry DOI: 10.7270/Q2WM1D8T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50048908
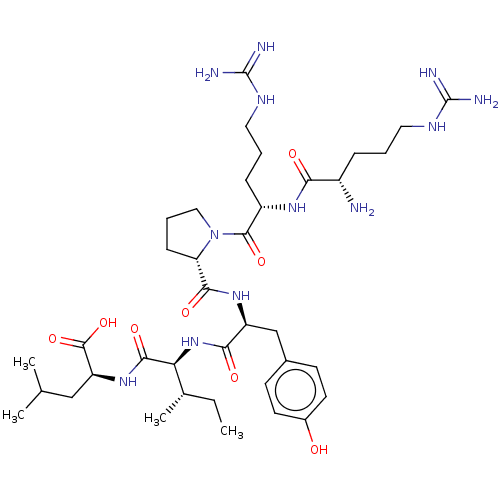
(CHEMBL415788)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22-,25-,26-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Angiotensin 2 from human placental AT1 receptor expressed in African green monkey COS7 cell membranes after 90 mins by gamma cou... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246648

((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...)Show SMILES CCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:8.8| Show InChI InChI=1S/C30H36N6O4/c1-2-32-30(40)36-29(31)33-19-9-14-25(27(38)34-20-21-15-17-24(37)18-16-21)35-28(39)26(22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-8,10-13,15-18,25-26,37H,2,9,14,19-20H2,1H3,(H,34,38)(H,35,39)(H4,31,32,33,36,40)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50532410

(CHEMBL4456247)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](N)CCCC[C@@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C80H124N26O16/c1-43(2)37-61(73(119)97-55(13-7-33-93-77(85)86)69(115)101-59(65(83)111)39-45-17-25-49(107)26-18-45)105-71(117)57(15-9-35-95-79(89)90)99-75(121)63(41-47-21-29-51(109)30-22-47)103-67(113)53(81)11-5-6-12-54(82)68(114)104-64(42-48-23-31-52(110)32-24-48)76(122)100-58(16-10-36-96-80(91)92)72(118)106-62(38-44(3)4)74(120)98-56(14-8-34-94-78(87)88)70(116)102-60(66(84)112)40-46-19-27-50(108)28-20-46/h17-32,43-44,53-64,107-110H,5-16,33-42,81-82H2,1-4H3,(H2,83,111)(H2,84,112)(H,97,119)(H,98,120)(H,99,121)(H,100,122)(H,101,115)(H,102,116)(H,103,113)(H,104,114)(H,105,117)(H,106,118)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t53-,54-,55+,56+,57+,58+,59+,60+,61+,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting |
J Med Chem 59: 6045-58 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00309
BindingDB Entry DOI: 10.7270/Q25T3Q08 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500151

(CHEMBL3746386)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCC(=O)NCCNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H41N7O5.2C2HF3O2/c1-2-28(42)35-20-21-37-33(45)40-32(34)36-19-9-14-27(30(43)38-22-23-15-17-26(41)18-16-23)39-31(44)29(24-10-5-3-6-11-24)25-12-7-4-8-13-25;2*3-2(4,5)1(6)7/h3-8,10-13,15-18,27,29,41H,2,9,14,19-22H2,1H3,(H,35,42)(H,38,43)(H,39,44)(H4,34,36,37,40,45);2*(H,6,7)/t27-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246648

((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...)Show SMILES CCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:8.8| Show InChI InChI=1S/C30H36N6O4/c1-2-32-30(40)36-29(31)33-19-9-14-25(27(38)34-20-21-15-17-24(37)18-16-21)35-28(39)26(22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-8,10-13,15-18,25-26,37H,2,9,14,19-20H2,1H3,(H,34,38)(H,35,39)(H4,31,32,33,36,40)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Asp-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-ValTyr-Ile-His-Pro-Phe-OH Tris(hydrotrifluoroacetate) from human AT1 re... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50343731

((R)-N-alpha-(2,2-Diphenylacetyl)-N-(4-ureidomethyl...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(-[#6]-[#7]-[#6](-[#7])=O)cc1 |r| Show InChI InChI=1S/C29H35N7O3/c30-28(31)33-17-7-12-24(26(37)34-18-20-13-15-21(16-14-20)19-35-29(32)39)36-27(38)25(22-8-3-1-4-9-22)23-10-5-2-6-11-23/h1-6,8-11,13-16,24-25H,7,12,17-19H2,(H,34,37)(H,36,38)(H4,30,31,33)(H3,32,35,39)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells |
Bioorg Med Chem 19: 2859-78 (2011)
Article DOI: 10.1016/j.bmc.2011.03.045
BindingDB Entry DOI: 10.7270/Q2F47PG7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50214615
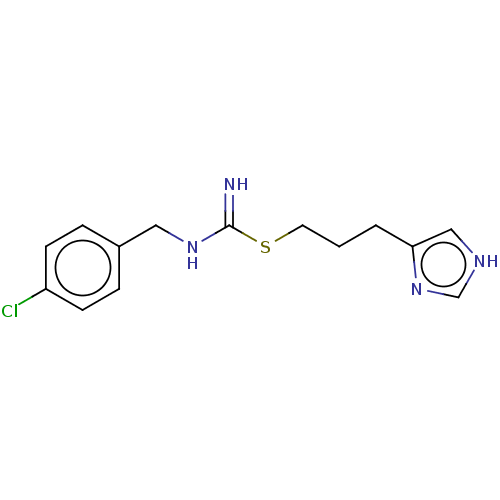
(CHEBI:64177 | Clobenpropit)Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of clobenpropit-BODIPY-630/650 from recombinant human NLuc-fused H3R expressed in HEK293T cells by Nano-BRET assay |
J Med Chem 63: 5297-5311 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00160
BindingDB Entry DOI: 10.7270/Q2WM1HZ1 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50159160

(CHEMBL3786779)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCN\C(N)=N\C(=O)NCCOCCOCCN)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C45H78N14O11.4C2HF3O2/c1-5-28(4)36(40(64)56-34(42(66)67)25-27(2)3)57-38(62)33(26-29-12-14-30(60)15-13-29)55-39(63)35-11-8-20-59(35)41(65)32(10-7-17-51-43(48)49)54-37(61)31(47)9-6-18-52-44(50)58-45(68)53-19-22-70-24-23-69-21-16-46;4*3-2(4,5)1(6)7/h12-15,27-28,31-36,60H,5-11,16-26,46-47H2,1-4H3,(H,54,61)(H,55,63)(H,56,64)(H,57,62)(H,66,67)(H4,48,49,51)(H4,50,52,53,58,68);4*(H,6,7)/t28-,31-,32-,33-,34-,35-,36-;;;;/m0..../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293T cells co-expressing CRE-Luc incubated for 60 mins by microbeta sci... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113190
BindingDB Entry DOI: 10.7270/Q2TF0245 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246648

((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...)Show SMILES CCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:8.8| Show InChI InChI=1S/C30H36N6O4/c1-2-32-30(40)36-29(31)33-19-9-14-25(27(38)34-20-21-15-17-24(37)18-16-21)35-28(39)26(22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-8,10-13,15-18,25-26,37H,2,9,14,19-20H2,1H3,(H,34,38)(H,35,39)(H4,31,32,33,36,40)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500154
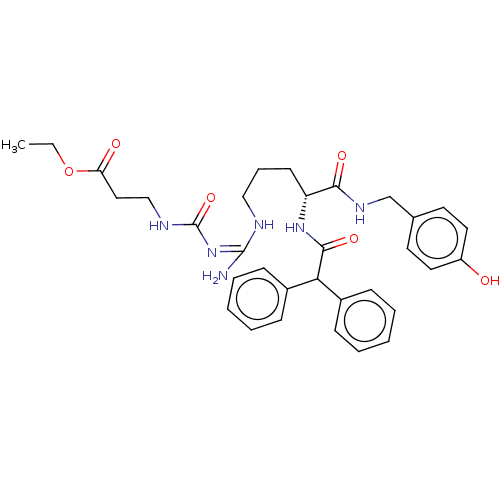
(CHEMBL3746870)Show SMILES CCOC(=O)CCNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H40N6O6/c1-2-45-28(41)19-21-36-33(44)39-32(34)35-20-9-14-27(30(42)37-22-23-15-17-26(40)18-16-23)38-31(43)29(24-10-5-3-6-11-24)25-12-7-4-8-13-25/h3-8,10-13,15-18,27,29,40H,2,9,14,19-22H2,1H3,(H,37,42)(H,38,43)(H4,34,35,36,39,44)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in HEL cells after 60 to 90 mins by flow cytometric analysis |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]propionyl-pNPY from NPY1R in HEL cells after 60 to 90 mins by flow cytometric analysis |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500151

(CHEMBL3746386)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCC(=O)NCCNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H41N7O5.2C2HF3O2/c1-2-28(42)35-20-21-37-33(45)40-32(34)36-19-9-14-27(30(43)38-22-23-15-17-26(41)18-16-23)39-31(44)29(24-10-5-3-6-11-24)25-12-7-4-8-13-25;2*3-2(4,5)1(6)7/h3-8,10-13,15-18,27,29,41H,2,9,14,19-22H2,1H3,(H,35,42)(H,38,43)(H,39,44)(H4,34,36,37,40,45);2*(H,6,7)/t27-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50535849
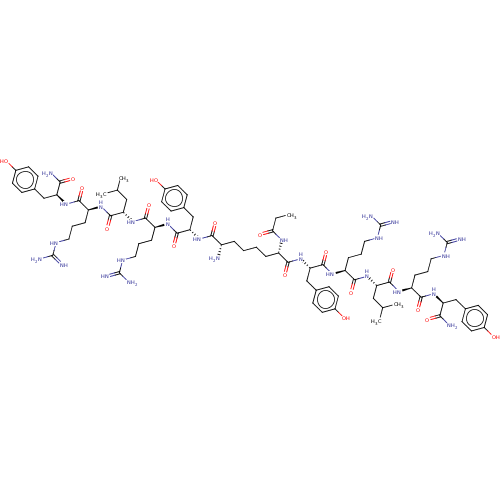
(CHEMBL4522438)Show SMILES CCC(=O)N[C@@H](CCCC[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C83H128N26O17/c1-6-67(114)99-56(71(118)109-66(44-50-25-33-54(113)34-26-50)79(126)103-60(18-12-38-98-83(93)94)75(122)108-64(40-46(4)5)77(124)101-58(16-10-36-96-81(89)90)73(120)105-62(69(86)116)42-48-21-29-52(111)30-22-48)14-8-7-13-55(84)70(117)106-65(43-49-23-31-53(112)32-24-49)78(125)102-59(17-11-37-97-82(91)92)74(121)107-63(39-45(2)3)76(123)100-57(15-9-35-95-80(87)88)72(119)104-61(68(85)115)41-47-19-27-51(110)28-20-47/h19-34,45-46,55-66,110-113H,6-18,35-44,84H2,1-5H3,(H2,85,115)(H2,86,116)(H,99,114)(H,100,123)(H,101,124)(H,102,125)(H,103,126)(H,104,119)(H,105,120)(H,106,117)(H,107,121)(H,108,122)(H,109,118)(H4,87,88,95)(H4,89,90,96)(H4,91,92,97)(H4,93,94,98)/t55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting |
J Med Chem 59: 6045-58 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00309
BindingDB Entry DOI: 10.7270/Q25T3Q08 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50214615

(CHEBI:64177 | Clobenpropit)Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H3R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... |
J Med Chem 63: 5297-5311 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00160
BindingDB Entry DOI: 10.7270/Q2WM1HZ1 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500153

(CHEMBL3746851)Show SMILES OC(=O)C(F)(F)F.Cn1cc(CNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)nn1 |r| Show InChI InChI=1S/C32H37N9O4.C2HF3O2/c1-41-21-25(39-40-41)20-36-32(45)38-31(33)34-18-8-13-27(29(43)35-19-22-14-16-26(42)17-15-22)37-30(44)28(23-9-4-2-5-10-23)24-11-6-3-7-12-24;3-2(4,5)1(6)7/h2-7,9-12,14-17,21,27-28,42H,8,13,18-20H2,1H3,(H,35,43)(H,37,44)(H4,33,34,36,38,45);(H,6,7)/t27-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50159205
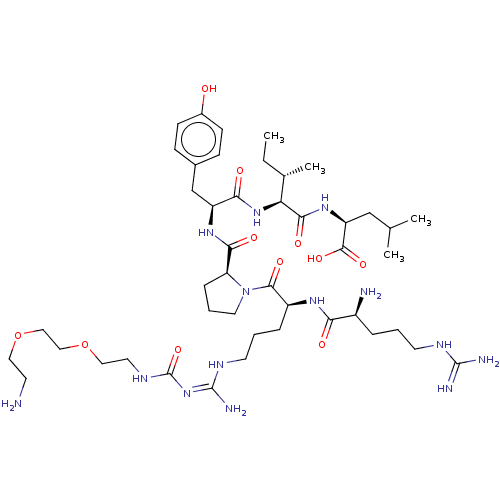
(CHEMBL3785233)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN\C(N)=N/C(=O)NCCOCCOCCN)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C45H78N14O11.4C2HF3O2/c1-5-28(4)36(40(64)56-34(42(66)67)25-27(2)3)57-38(62)33(26-29-12-14-30(60)15-13-29)55-39(63)35-11-8-20-59(35)41(65)32(54-37(61)31(47)9-6-17-51-43(48)49)10-7-18-52-44(50)58-45(68)53-19-22-70-24-23-69-21-16-46;4*3-2(4,5)1(6)7/h12-15,27-28,31-36,60H,5-11,16-26,46-47H2,1-4H3,(H,54,61)(H,55,63)(H,56,64)(H,57,62)(H,66,67)(H4,48,49,51)(H4,50,52,53,58,68);4*(H,6,7)/t28-,31-,32-,33-,34-,35-,36-;;;;/m0..../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50528183

(CHEMBL4540843)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C185H287N53O54S2/c1-20-92(10)144(175(286)228-125(84-137(190)248)164(275)215-115(64-75-294-19)159(270)222-121(78-90(6)7)167(278)232-145(98(16)239)176(287)219-116(33-24-68-203-185(198)199)178(289)236-71-27-36-131(236)170(281)216-110(32-23-67-202-184(196)197)154(265)220-118(147(191)258)79-100-39-47-104(241)48-40-100)231-168(279)123(81-102-43-51-106(243)52-44-102)225-155(266)109(31-22-66-201-183(194)195)211-153(264)108(30-21-65-200-182(192)193)212-162(273)119(76-88(2)3)223-166(277)127(86-142(256)257)221-150(261)95(13)205-148(259)94(12)207-160(271)122(80-101-41-49-105(242)50-42-101)224-158(269)111(55-59-134(187)245)210-149(260)96(14)206-152(263)114(63-74-293-18)214-156(267)112(56-60-135(188)246)213-157(268)113(57-61-139(250)251)217-171(282)132-37-29-73-238(132)181(292)146(99(17)240)233-151(262)97(15)208-161(272)124(83-136(189)247)226-165(276)126(85-141(254)255)209-138(249)87-204-169(280)129-34-25-70-235(129)180(291)128(82-103-45-53-107(244)54-46-103)229-174(285)143(91(8)9)230-173(284)133-38-28-72-237(133)179(290)117(58-62-140(252)253)218-163(274)120(77-89(4)5)227-172(283)130-35-26-69-234(130)177(288)93(11)186/h39-54,88-99,108-133,143-146,239-244H,20-38,55-87,186H2,1-19H3,(H2,187,245)(H2,188,246)(H2,189,247)(H2,190,248)(H2,191,258)(H,204,280)(H,205,259)(H,206,263)(H,207,271)(H,208,272)(H,209,249)(H,210,260)(H,211,264)(H,212,273)(H,213,268)(H,214,267)(H,215,275)(H,216,281)(H,217,282)(H,218,274)(H,219,287)(H,220,265)(H,221,261)(H,222,270)(H,223,277)(H,224,269)(H,225,266)(H,226,276)(H,227,283)(H,228,286)(H,229,285)(H,230,284)(H,231,279)(H,232,278)(H,233,262)(H,250,251)(H,252,253)(H,254,255)(H,256,257)(H4,192,193,200)(H4,194,195,201)(H4,196,197,202)(H4,198,199,203)/t92-,93-,94-,95-,96-,97-,98+,99+,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,143-,144-,145-,146-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting |
J Med Chem 59: 6045-58 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00309
BindingDB Entry DOI: 10.7270/Q25T3Q08 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246648

((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...)Show SMILES CCNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:8.8| Show InChI InChI=1S/C30H36N6O4/c1-2-32-30(40)36-29(31)33-19-9-14-25(27(38)34-20-21-15-17-24(37)18-16-21)35-28(39)26(22-10-5-3-6-11-22)23-12-7-4-8-13-23/h3-8,10-13,15-18,25-26,37H,2,9,14,19-20H2,1H3,(H,34,38)(H,35,39)(H4,31,32,33,36,40)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50159164
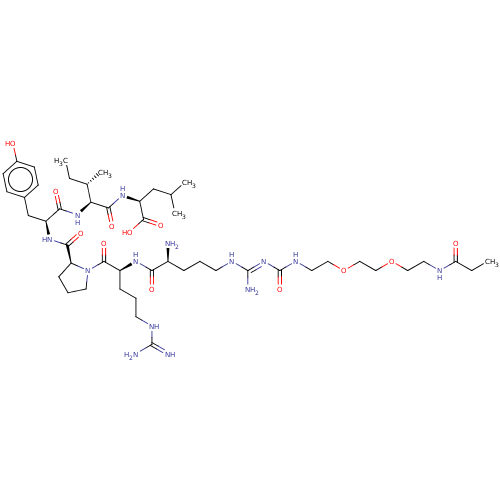
(CHEMBL3787200)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCN\C(N)=N\C(=O)NCCOCCOCCNC(=O)CC)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C48H82N14O12.3C2HF3O2/c1-6-30(5)39(43(68)59-36(45(70)71)27-29(3)4)60-41(66)35(28-31-14-16-32(63)17-15-31)58-42(67)37-13-10-22-62(37)44(69)34(12-9-18-54-46(50)51)57-40(65)33(49)11-8-19-55-47(52)61-48(72)56-21-24-74-26-25-73-23-20-53-38(64)7-2;3*3-2(4,5)1(6)7/h14-17,29-30,33-37,39,63H,6-13,18-28,49H2,1-5H3,(H,53,64)(H,57,65)(H,58,67)(H,59,68)(H,60,66)(H,70,71)(H4,50,51,54)(H4,52,55,56,61,72);3*(H,6,7)/t30-,33-,34-,35-,36-,37-,39-;;;/m0.../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500157
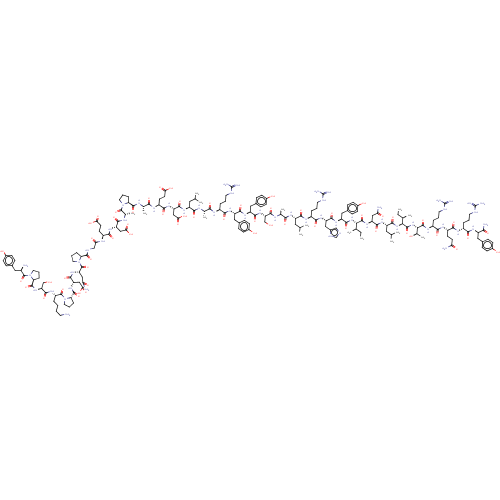
(CHEMBL4299523)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C190H287N55O57/c1-16-94(9)149(180(296)235-129(81-141(194)255)169(285)227-124(74-93(7)8)172(288)240-150(95(10)17-2)181(297)241-151(100(15)248)182(298)222-116(32-23-67-209-190(203)204)156(272)221-118(57-60-140(193)254)161(277)219-114(30-21-65-207-188(199)200)157(273)224-121(152(196)268)76-102-39-49-108(250)50-40-102)239-173(289)127(79-105-45-55-111(253)56-46-105)230-168(284)128(80-106-86-205-90-211-106)231-159(275)115(31-22-66-208-189(201)202)220-165(281)123(73-92(5)6)225-155(271)97(12)213-174(290)134(88-246)237-167(283)126(78-104-43-53-110(252)54-44-104)229-166(282)125(77-103-41-51-109(251)52-42-103)228-158(274)113(29-20-64-206-187(197)198)217-153(269)96(11)212-163(279)122(72-91(3)4)226-170(286)131(84-147(264)265)233-162(278)119(59-62-145(260)261)218-154(270)98(13)214-177(293)137-34-25-68-242(137)183(299)99(14)215-164(280)130(83-146(262)263)232-160(276)117(58-61-144(258)259)216-143(257)87-210-176(292)136-33-24-70-244(136)186(302)133(82-142(195)256)236-171(287)132(85-148(266)267)234-178(294)139-36-27-71-245(139)185(301)120(28-18-19-63-191)223-175(291)135(89-247)238-179(295)138-35-26-69-243(138)184(300)112(192)75-101-37-47-107(249)48-38-101/h37-56,86,90-100,112-139,149-151,246-253H,16-36,57-85,87-89,191-192H2,1-15H3,(H2,193,254)(H2,194,255)(H2,195,256)(H2,196,268)(H,205,211)(H,210,292)(H,212,279)(H,213,290)(H,214,293)(H,215,280)(H,216,257)(H,217,269)(H,218,270)(H,219,277)(H,220,281)(H,221,272)(H,222,298)(H,223,291)(H,224,273)(H,225,271)(H,226,286)(H,227,285)(H,228,274)(H,229,282)(H,230,284)(H,231,275)(H,232,276)(H,233,278)(H,234,294)(H,235,296)(H,236,287)(H,237,283)(H,238,295)(H,239,289)(H,240,288)(H,241,297)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H4,197,198,206)(H4,199,200,207)(H4,201,202,208)(H4,203,204,209)/t94-,95-,96+,97+,98+,99+,100-,112-,113+,114-,115+,116+,117+,118+,119+,120+,121-,122+,123+,124+,125+,126+,127+,128+,129+,130+,131+,132+,133+,134+,135+,136+,137+,138+,139+,149+,150+,151+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50246649

((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...)Show SMILES CCOC(=O)CNC(=O)NC(N)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:12.12| Show InChI InChI=1S/C32H38N6O6/c1-2-44-27(40)21-36-32(43)38-31(33)34-19-9-14-26(29(41)35-20-22-15-17-25(39)18-16-22)37-30(42)28(23-10-5-3-6-11-23)24-12-7-4-8-13-24/h3-8,10-13,15-18,26,28,39H,2,9,14,19-21H2,1H3,(H,35,41)(H,37,42)(H4,33,34,36,38,43)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500157

(CHEMBL4299523)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C190H287N55O57/c1-16-94(9)149(180(296)235-129(81-141(194)255)169(285)227-124(74-93(7)8)172(288)240-150(95(10)17-2)181(297)241-151(100(15)248)182(298)222-116(32-23-67-209-190(203)204)156(272)221-118(57-60-140(193)254)161(277)219-114(30-21-65-207-188(199)200)157(273)224-121(152(196)268)76-102-39-49-108(250)50-40-102)239-173(289)127(79-105-45-55-111(253)56-46-105)230-168(284)128(80-106-86-205-90-211-106)231-159(275)115(31-22-66-208-189(201)202)220-165(281)123(73-92(5)6)225-155(271)97(12)213-174(290)134(88-246)237-167(283)126(78-104-43-53-110(252)54-44-104)229-166(282)125(77-103-41-51-109(251)52-42-103)228-158(274)113(29-20-64-206-187(197)198)217-153(269)96(11)212-163(279)122(72-91(3)4)226-170(286)131(84-147(264)265)233-162(278)119(59-62-145(260)261)218-154(270)98(13)214-177(293)137-34-25-68-242(137)183(299)99(14)215-164(280)130(83-146(262)263)232-160(276)117(58-61-144(258)259)216-143(257)87-210-176(292)136-33-24-70-244(136)186(302)133(82-142(195)256)236-171(287)132(85-148(266)267)234-178(294)139-36-27-71-245(139)185(301)120(28-18-19-63-191)223-175(291)135(89-247)238-179(295)138-35-26-69-243(138)184(300)112(192)75-101-37-47-107(249)48-38-101/h37-56,86,90-100,112-139,149-151,246-253H,16-36,57-85,87-89,191-192H2,1-15H3,(H2,193,254)(H2,194,255)(H2,195,256)(H2,196,268)(H,205,211)(H,210,292)(H,212,279)(H,213,290)(H,214,293)(H,215,280)(H,216,257)(H,217,269)(H,218,270)(H,219,277)(H,220,281)(H,221,272)(H,222,298)(H,223,291)(H,224,273)(H,225,271)(H,226,286)(H,227,285)(H,228,274)(H,229,282)(H,230,284)(H,231,275)(H,232,276)(H,233,278)(H,234,294)(H,235,296)(H,236,287)(H,237,283)(H,238,295)(H,239,289)(H,240,288)(H,241,297)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H4,197,198,206)(H4,199,200,207)(H4,201,202,208)(H4,203,204,209)/t94-,95-,96+,97+,98+,99+,100-,112-,113+,114-,115+,116+,117+,118+,119+,120+,121-,122+,123+,124+,125+,126+,127+,128+,129+,130+,131+,132+,133+,134+,135+,136+,137+,138+,139+,149+,150+,151+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50532410

(CHEMBL4456247)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](N)CCCC[C@@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C80H124N26O16/c1-43(2)37-61(73(119)97-55(13-7-33-93-77(85)86)69(115)101-59(65(83)111)39-45-17-25-49(107)26-18-45)105-71(117)57(15-9-35-95-79(89)90)99-75(121)63(41-47-21-29-51(109)30-22-47)103-67(113)53(81)11-5-6-12-54(82)68(114)104-64(42-48-23-31-52(110)32-24-48)76(122)100-58(16-10-36-96-80(91)92)72(118)106-62(38-44(3)4)74(120)98-56(14-8-34-94-78(87)88)70(116)102-60(66(84)112)40-46-19-27-50(108)28-20-46/h17-32,43-44,53-64,107-110H,5-16,33-42,81-82H2,1-4H3,(H2,83,111)(H2,84,112)(H,97,119)(H,98,120)(H,99,121)(H,100,122)(H,101,115)(H,102,116)(H,103,113)(H,104,114)(H,105,117)(H,106,118)(H4,85,86,93)(H4,87,88,94)(H4,89,90,95)(H4,91,92,96)/t53-,54-,55+,56+,57+,58+,59+,60+,61+,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H](2R,7R)-10 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins in presence of sodium-free HE... |
J Med Chem 59: 6045-58 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00309
BindingDB Entry DOI: 10.7270/Q25T3Q08 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50159155
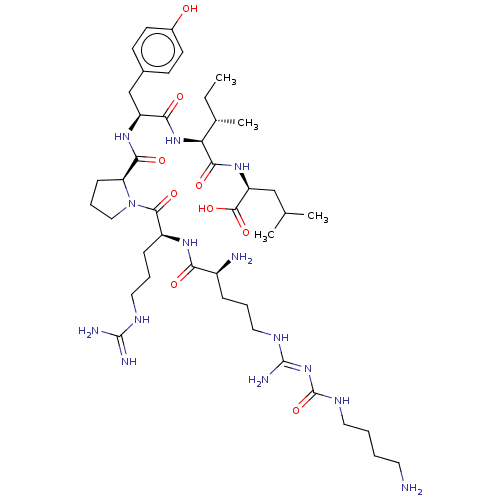
(CHEMBL3786852)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCN\C(N)=N\C(=O)NCCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C43H74N14O9.4C2HF3O2/c1-5-26(4)34(38(62)54-32(40(64)65)23-25(2)3)55-36(60)31(24-27-14-16-28(58)17-15-27)53-37(61)33-13-10-22-57(33)39(63)30(12-9-20-49-41(46)47)52-35(59)29(45)11-8-21-50-42(48)56-43(66)51-19-7-6-18-44;4*3-2(4,5)1(6)7/h14-17,25-26,29-34,58H,5-13,18-24,44-45H2,1-4H3,(H,52,59)(H,53,61)(H,54,62)(H,55,60)(H,64,65)(H4,46,47,49)(H4,48,50,51,56,66);4*(H,6,7)/t26-,29-,30-,31-,32-,33-,34-;;;;/m0..../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50159156

(CHEMBL3786291)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCN\C(N)=N\C(=O)NCCCCNC(=O)CC)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C46H78N14O10.3C2HF3O2/c1-6-28(5)37(41(66)57-34(43(68)69)25-27(3)4)58-39(64)33(26-29-16-18-30(61)19-17-29)56-40(65)35-15-12-24-60(35)42(67)32(14-11-22-52-44(48)49)55-38(63)31(47)13-10-23-53-45(50)59-46(70)54-21-9-8-20-51-36(62)7-2;3*3-2(4,5)1(6)7/h16-19,27-28,31-35,37,61H,6-15,20-26,47H2,1-5H3,(H,51,62)(H,55,63)(H,56,65)(H,57,66)(H,58,64)(H,68,69)(H4,48,49,52)(H4,50,53,54,59,70);3*(H,6,7)/t28-,31-,32-,33-,34-,35-,37-;;;/m0.../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500154

(CHEMBL3746870)Show SMILES CCOC(=O)CCNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H40N6O6/c1-2-45-28(41)19-21-36-33(44)39-32(34)35-20-9-14-27(30(42)37-22-23-15-17-26(40)18-16-23)38-31(43)29(24-10-5-3-6-11-24)25-12-7-4-8-13-25/h3-8,10-13,15-18,27,29,40H,2,9,14,19-22H2,1H3,(H,37,42)(H,38,43)(H4,34,35,36,39,44)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK136 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50005530
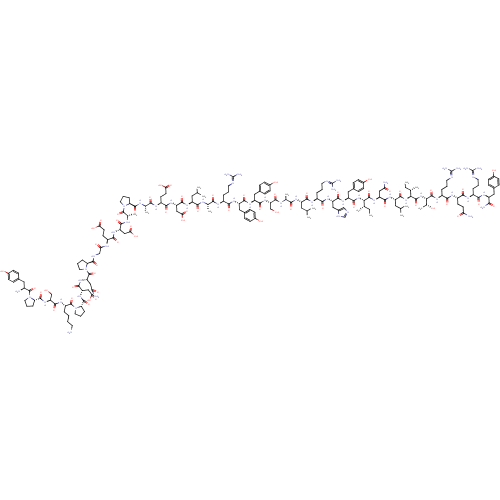
(CHEMBL267633 | D-Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-G...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C190H287N55O57/c1-16-94(9)149(180(296)235-129(81-141(194)255)169(285)227-124(74-93(7)8)172(288)240-150(95(10)17-2)181(297)241-151(100(15)248)182(298)222-116(32-23-67-209-190(203)204)156(272)221-118(57-60-140(193)254)161(277)219-114(30-21-65-207-188(199)200)157(273)224-121(152(196)268)76-102-39-49-108(250)50-40-102)239-173(289)127(79-105-45-55-111(253)56-46-105)230-168(284)128(80-106-86-205-90-211-106)231-159(275)115(31-22-66-208-189(201)202)220-165(281)123(73-92(5)6)225-155(271)97(12)213-174(290)134(88-246)237-167(283)126(78-104-43-53-110(252)54-44-104)229-166(282)125(77-103-41-51-109(251)52-42-103)228-158(274)113(29-20-64-206-187(197)198)217-153(269)96(11)212-163(279)122(72-91(3)4)226-170(286)131(84-147(264)265)233-162(278)119(59-62-145(260)261)218-154(270)98(13)214-177(293)137-34-25-68-242(137)183(299)99(14)215-164(280)130(83-146(262)263)232-160(276)117(58-61-144(258)259)216-143(257)87-210-176(292)136-33-24-70-244(136)186(302)133(82-142(195)256)236-171(287)132(85-148(266)267)234-178(294)139-36-27-71-245(139)185(301)120(28-18-19-63-191)223-175(291)135(89-247)238-179(295)138-35-26-69-243(138)184(300)112(192)75-101-37-47-107(249)48-38-101/h37-56,86,90-100,112-139,149-151,246-253H,16-36,57-85,87-89,191-192H2,1-15H3,(H2,193,254)(H2,194,255)(H2,195,256)(H2,196,268)(H,205,211)(H,210,292)(H,212,279)(H,213,290)(H,214,293)(H,215,280)(H,216,257)(H,217,269)(H,218,270)(H,219,277)(H,220,281)(H,221,272)(H,222,298)(H,223,291)(H,224,273)(H,225,271)(H,226,286)(H,227,285)(H,228,274)(H,229,282)(H,230,284)(H,231,275)(H,232,276)(H,233,278)(H,234,294)(H,235,296)(H,236,287)(H,237,283)(H,238,295)(H,239,289)(H,240,288)(H,241,297)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,266,267)(H4,197,198,206)(H4,199,200,207)(H4,201,202,208)(H4,203,204,209)/t94-,95-,96-,97-,98-,99-,100+,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,149-,150-,151-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells |
J Med Chem 51: 8168-72 (2008)
Article DOI: 10.1021/jm801018u
BindingDB Entry DOI: 10.7270/Q2WM1D8T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50159232
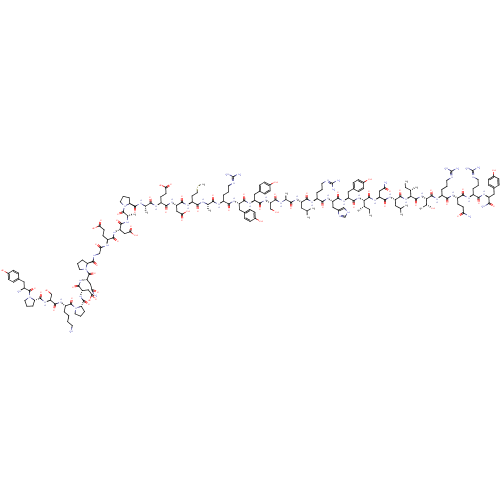
(CHEMBL3786263)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCCNC(N)=N)NC(=O)C(CC(C)C)NC(=O)C(CC(O)=O)NC(=O)C(C)NC(=O)C(C)NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(CCC(N)=O)NC(=O)C(C)NC(=O)C(CCSC)NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C1CCCN1C(=O)C(NC(=O)C(C)NC(=O)C(CC(N)=O)NC(=O)C(CC(O)=O)NC(=O)CNC(=O)C1CCCN1C(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C1CCCN1C(=O)C(CCC(O)=O)NC(=O)C(CC(C)C)NC(=O)C1CCCN1C(=O)C(C)N)C(C)C)C(C)O)C(=O)NC(CC(N)=O)C(=O)NC(CCSC)C(=O)NC(CC(C)C)C(=O)NC(C(C)O)C(=O)NC(CCCNC(N)=N)C(=O)N1CCCC1C(=O)NC(CCCNC(N)=N)C(=O)NC(Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C185H286N52O55S2/c1-20-92(10)144(174(284)226-124(84-137(190)247)163(273)214-115(64-75-294-19)158(268)220-120(78-90(6)7)166(276)231-145(98(16)238)175(285)218-116(33-24-68-202-185(197)198)177(287)235-71-27-36-131(235)169(279)215-110(32-23-67-201-184(195)196)154(264)228-128(181(291)292)82-103-45-53-107(243)54-46-103)230-167(277)122(80-101-41-49-105(241)50-42-101)223-153(263)109(31-22-66-200-183(193)194)210-152(262)108(30-21-65-199-182(191)192)211-161(271)118(76-88(2)3)221-165(275)126(86-142(255)256)219-149(259)95(13)204-147(257)94(12)206-159(269)121(79-100-39-47-104(240)48-40-100)222-157(267)111(55-59-134(187)244)209-148(258)96(14)205-151(261)114(63-74-293-18)213-155(265)112(56-60-135(188)245)212-156(266)113(57-61-139(249)250)216-170(280)132-37-29-73-237(132)180(290)146(99(17)239)232-150(260)97(15)207-160(270)123(83-136(189)246)224-164(274)125(85-141(253)254)208-138(248)87-203-168(278)129-34-25-70-234(129)179(289)127(81-102-43-51-106(242)52-44-102)227-173(283)143(91(8)9)229-172(282)133-38-28-72-236(133)178(288)117(58-62-140(251)252)217-162(272)119(77-89(4)5)225-171(281)130-35-26-69-233(130)176(286)93(11)186/h39-54,88-99,108-133,143-146,238-243H,20-38,55-87,186H2,1-19H3,(H2,187,244)(H2,188,245)(H2,189,246)(H2,190,247)(H,203,278)(H,204,257)(H,205,261)(H,206,269)(H,207,270)(H,208,248)(H,209,258)(H,210,262)(H,211,271)(H,212,266)(H,213,265)(H,214,273)(H,215,279)(H,216,280)(H,217,272)(H,218,285)(H,219,259)(H,220,268)(H,221,275)(H,222,267)(H,223,263)(H,224,274)(H,225,281)(H,226,284)(H,227,283)(H,228,264)(H,229,282)(H,230,277)(H,231,276)(H,232,260)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,291,292)(H4,191,192,199)(H4,193,194,200)(H4,195,196,201)(H4,197,198,202)/t92-,93-,94-,95-,96-,97-,98+,99-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,143-,144-,145-,146-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of S0586[K4]hpp from human NPY4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ by flow cytometric analysis |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50015490
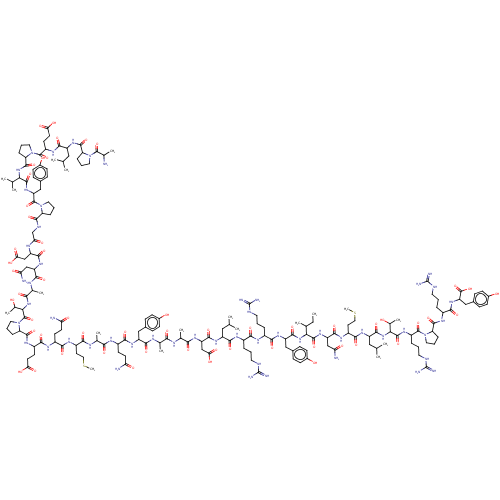
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Cy5-pNPY from human Y1 receptor expressed in HEL cells after 90 to 120 mins by flow cytometry |
J Med Chem 56: 8422-31 (2013)
Article DOI: 10.1021/jm4008505
BindingDB Entry DOI: 10.7270/Q25X2BC9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50150945
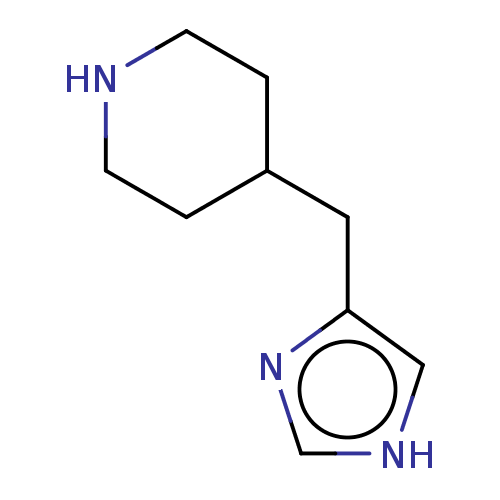
(CHEBI:81390 | Immepip)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells |
Bioorg Med Chem Lett 20: 7191-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.041
BindingDB Entry DOI: 10.7270/Q2Q81GX5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50214615

(CHEBI:64177 | Clobenpropit)Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranes co... |
J Med Chem 63: 5297-5311 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00160
BindingDB Entry DOI: 10.7270/Q2WM1HZ1 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM50159227
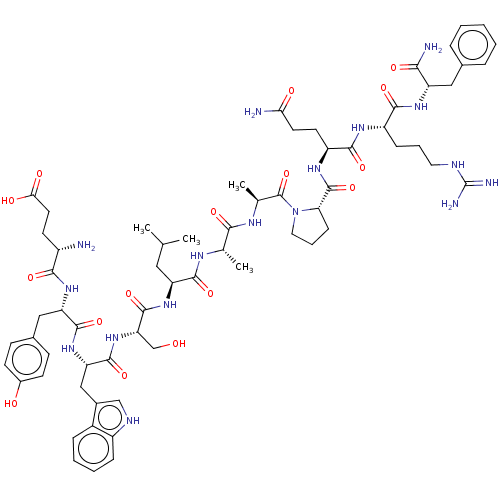
(CHEMBL3786309)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C65H91N17O16/c1-34(2)28-47(79-62(96)50(33-83)81-61(95)49(31-39-32-72-43-15-9-8-14-41(39)43)80-60(94)48(30-38-18-20-40(84)21-19-38)78-56(90)42(66)22-25-53(86)87)59(93)73-35(3)55(89)74-36(4)64(98)82-27-11-17-51(82)63(97)76-45(23-24-52(67)85)58(92)75-44(16-10-26-71-65(69)70)57(91)77-46(54(68)88)29-37-12-6-5-7-13-37/h5-9,12-15,18-21,32,34-36,42,44-51,72,83-84H,10-11,16-17,22-31,33,66H2,1-4H3,(H2,67,85)(H2,68,88)(H,73,93)(H,74,89)(H,75,92)(H,76,97)(H,77,91)(H,78,90)(H,79,96)(H,80,94)(H,81,95)(H,86,87)(H4,69,70,71)/t35-,36-,42-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]EYE from human NPFF2 receptor expressed in CHO cell membranes after 1 hr by liquid scintillation spectrophotometric counting |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50441964
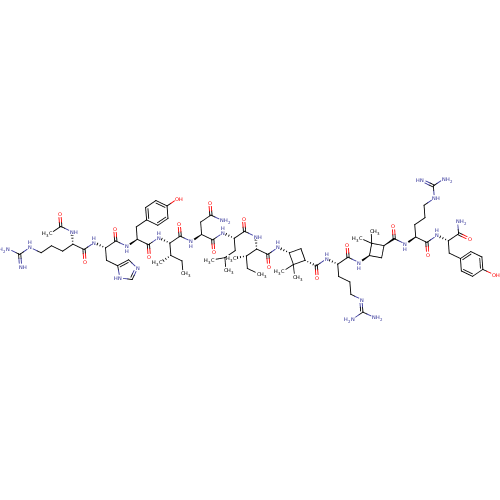
(CHEMBL2440193)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCCN=C(N)N)NC(C)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]1C[C@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]2C[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3ccc(O)cc3)C(N)=O)C2(C)C)C1(C)C |r,wU:4.4,30.39,86.87,88.90,61.62,71.73,69.70,53.54,8.17,wD:62.64,103.104,20.28,2.2,75.76,45.46,92.93,(28.14,3.58,;26.8,2.79,;26.8,1.28,;28.14,.5,;25.45,.5,;24.13,1.26,;22.82,.5,;22.82,-1.03,;21.47,1.28,;21.47,2.84,;22.82,3.61,;24.15,2.81,;25.51,3.55,;25.52,5.11,;26.85,5.87,;24.18,5.88,;22.83,5.12,;20.15,.52,;18.81,1.29,;18.81,2.85,;17.49,.53,;17.49,-1,;16.14,-1.78,;15.96,-3.29,;14.44,-3.6,;13.68,-2.26,;14.73,-1.12,;16.14,1.3,;16.14,2.86,;17.49,3.64,;14.83,3.62,;14.83,5.18,;13.51,5.94,;13.51,7.49,;12.19,8.26,;12.19,9.8,;13.53,10.59,;10.87,10.56,;13.51,2.86,;12.17,3.63,;10.84,2.86,;12.17,5.18,;25.45,-1.03,;26.8,-1.81,;24.11,-1.81,;24.11,-3.36,;22.79,-4.13,;21.44,-3.35,;20.13,-4.11,;21.44,-1.79,;25.45,-4.14,;25.45,-5.66,;26.77,-3.38,;28.1,-4.14,;29.44,-3.37,;30.78,-4.15,;30.78,-5.7,;32.1,-3.38,;28.1,-5.68,;26.76,-6.45,;29.43,-6.45,;29.43,-8,;30.75,-8.76,;30.75,-10.31,;32.07,-8,;32.07,-6.48,;28.09,-8.77,;26.74,-8,;28.09,-10.3,;26.74,-11.07,;25.33,-10.68,;24.82,-12.11,;23.43,-12.78,;22.15,-11.92,;23.32,-14.31,;21.94,-14.99,;20.66,-14.13,;20.77,-12.59,;19.5,-11.73,;19.6,-10.2,;18.33,-9.34,;18.43,-7.8,;16.95,-10.01,;21.83,-16.52,;23.11,-17.38,;20.45,-17.2,;20.34,-18.73,;19.17,-19.74,;20.1,-20.9,;19.9,-22.42,;18.53,-23.1,;21.18,-23.28,;21.07,-24.82,;19.69,-25.49,;18.41,-24.63,;17.04,-25.31,;16.93,-26.84,;15.55,-27.52,;14.27,-26.65,;15.44,-29.05,;22.35,-25.68,;22.24,-27.21,;23.73,-25,;25.01,-25.86,;24.9,-27.4,;26.18,-28.25,;26.07,-29.79,;27.34,-30.65,;28.73,-29.99,;30,-30.86,;28.84,-28.45,;27.56,-27.59,;26.39,-25.19,;27.67,-26.05,;26.5,-23.65,;21.37,-19.92,;22.55,-18.93,;22.33,-21.11,;26.3,-12.64,;25.82,-14.1,;27.77,-13.09,)| Show InChI InChI=1S/C80H127N25O16/c1-12-41(5)62(104-73(119)56(33-45-22-26-48(108)27-23-45)99-70(116)57(34-46-38-89-39-93-46)100-67(113)51(94-43(7)106)17-14-28-90-76(83)84)74(120)101-58(37-61(81)109)71(117)98-55(31-40(3)4)72(118)105-63(42(6)13-2)75(121)103-60-36-50(80(60,10)11)66(112)96-53(19-16-30-92-78(87)88)69(115)102-59-35-49(79(59,8)9)65(111)95-52(18-15-29-91-77(85)86)68(114)97-54(64(82)110)32-44-20-24-47(107)25-21-44/h20-27,38-42,49-60,62-63,107-108H,12-19,28-37H2,1-11H3,(H2,81,109)(H2,82,110)(H,89,93)(H,94,106)(H,95,111)(H,96,112)(H,97,114)(H,98,117)(H,99,116)(H,100,113)(H,101,120)(H,102,115)(H,103,121)(H,104,119)(H,105,118)(H4,83,84,90)(H4,85,86,91)(H4,87,88,92)/t41-,42-,49+,50+,51-,52-,53-,54-,55-,56-,57-,58-,59+,60+,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of Cy5-[K4]hPP from human Y4 receptor expressed in CHO cells after 90 to 120 mins by flow cytometry |
J Med Chem 56: 8422-31 (2013)
Article DOI: 10.1021/jm4008505
BindingDB Entry DOI: 10.7270/Q25X2BC9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50441965
(CHEMBL2440192)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#16]-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#16]-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7])-[#6](-[#6])-[#6])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-[#6@H](-[#7])-[#6]-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C184H287N53O53S2/c1-20-92(10)144(174(284)218-116(33-24-68-202-184(197)198)177(287)235-71-27-36-130(235)169(279)215-110(32-23-67-201-183(195)196)158(268)228-133(186)82-103-45-53-107(243)54-46-103)230-166(276)120(78-90(6)7)220-157(267)115(64-75-292-19)214-163(273)124(84-137(190)247)226-175(285)145(98(16)238)231-167(277)122(80-101-41-49-105(241)50-42-101)223-153(263)109(31-22-66-200-182(193)194)210-152(262)108(30-21-65-199-181(191)192)211-161(271)118(76-88(2)3)221-165(275)126(86-142(255)256)219-149(259)95(13)204-147(257)94(12)206-159(269)121(79-100-39-47-104(240)48-40-100)222-156(266)111(55-59-134(187)244)209-148(258)96(14)205-151(261)114(63-74-291-18)213-154(264)112(56-60-135(188)245)212-155(265)113(57-61-139(249)250)216-170(280)131-37-29-73-237(131)180(290)146(99(17)239)232-150(260)97(15)207-160(270)123(83-136(189)246)224-164(274)125(85-141(253)254)208-138(248)87-203-168(278)128-34-25-70-234(128)179(289)127(81-102-43-51-106(242)52-44-102)227-173(283)143(91(8)9)229-172(282)132-38-28-72-236(132)178(288)117(58-62-140(251)252)217-162(272)119(77-89(4)5)225-171(281)129-35-26-69-233(129)176(286)93(11)185/h39-54,88-99,108-133,143-146,238-243H,20-38,55-87,185-186H2,1-19H3,(H2,187,244)(H2,188,245)(H2,189,246)(H2,190,247)(H,203,278)(H,204,257)(H,205,261)(H,206,269)(H,207,270)(H,208,248)(H,209,258)(H,210,262)(H,211,271)(H,212,265)(H,213,264)(H,214,273)(H,215,279)(H,216,280)(H,217,272)(H,218,284)(H,219,259)(H,220,267)(H,221,275)(H,222,266)(H,223,263)(H,224,274)(H,225,281)(H,226,285)(H,227,283)(H,228,268)(H,229,282)(H,230,276)(H,231,277)(H,232,260)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H4,191,192,199)(H4,193,194,200)(H4,195,196,201)(H4,197,198,202)/t92-,93-,94-,95-,96-,97-,98+,99+,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,143-,144-,145-,146-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Binding affinity to human Y4 receptor |
J Med Chem 56: 8422-31 (2013)
Article DOI: 10.1021/jm4008505
BindingDB Entry DOI: 10.7270/Q25X2BC9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50538682
(CHEMBL4597218)Show InChI InChI=1S/C15H22N6/c1-15(2,3)9-17-14-16-6-4-13(20-14)21-7-5-11-12(8-21)19-10-18-11/h4,6,10H,5,7-9H2,1-3H3,(H,18,19)(H,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H3R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... |
J Med Chem 63: 5297-5311 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00160
BindingDB Entry DOI: 10.7270/Q2WM1HZ1 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50500153

(CHEMBL3746851)Show SMILES OC(=O)C(F)(F)F.Cn1cc(CNC(=O)\N=C(\N)NCCC[C@@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCc2ccc(O)cc2)nn1 |r| Show InChI InChI=1S/C32H37N9O4.C2HF3O2/c1-41-21-25(39-40-41)20-36-32(45)38-31(33)34-18-8-13-27(29(43)35-19-22-14-16-26(42)17-15-22)37-30(44)28(23-9-4-2-5-10-23)24-11-6-3-7-12-24;3-2(4,5)1(6)7/h2-7,9-12,14-17,21,27-28,42H,8,13,18-20H2,1H3,(H,35,43)(H,37,44)(H4,33,34,36,38,45);(H,6,7)/t27-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale)
Curated by ChEMBL
| Assay Description
Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay |
J Med Chem 58: 8834-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00925
BindingDB Entry DOI: 10.7270/Q2RN3BVG |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50159212

(CHEMBL3786771)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN\C(N)=N/C(=O)NCCOCCOCCNC(=O)CC)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C48H82N14O12.3C2HF3O2/c1-6-30(5)39(43(68)59-36(45(70)71)27-29(3)4)60-41(66)35(28-31-14-16-32(63)17-15-31)58-42(67)37-13-10-22-62(37)44(69)34(57-40(65)33(49)11-8-18-54-46(50)51)12-9-19-55-47(52)61-48(72)56-21-24-74-26-25-73-23-20-53-38(64)7-2;3*3-2(4,5)1(6)7/h14-17,29-30,33-37,39,63H,6-13,18-28,49H2,1-5H3,(H,53,64)(H,57,65)(H,58,67)(H,59,68)(H,60,66)(H,70,71)(H4,50,51,54)(H4,52,55,56,61,72);3*(H,6,7)/t30-,33-,34-,35-,36-,37-,39-;;;/m0.../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... |
J Med Chem 59: 1925-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01495
BindingDB Entry DOI: 10.7270/Q2V69MGK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































