

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50161342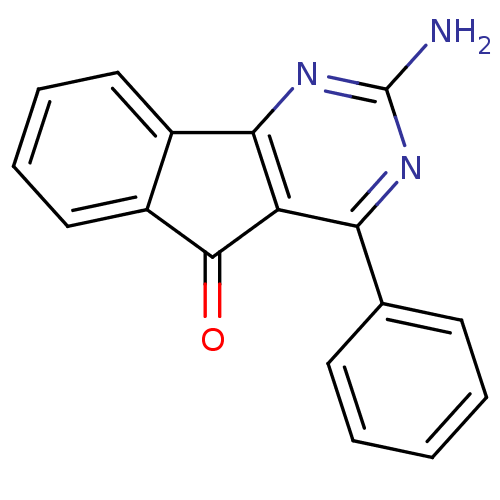 (2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from A1 adenosine receptor (unknown origin) expressed in CHO cell membrane incubated for 60 mins at room temperature by NXT... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408573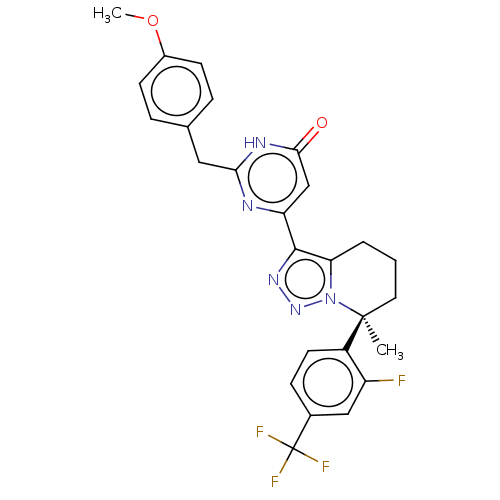 (US10358435, Example 89 | US10358435, Example 90) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408558 (US10358435, Example 74 | US10358435, Example 75) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50268107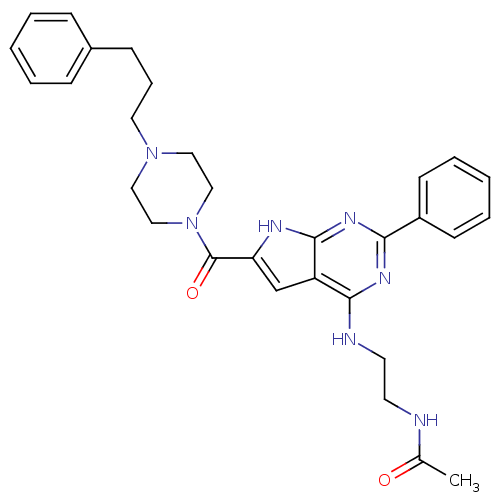 (CHEMBL485862 | CHEMBL500634 | N-(2-(2-phenyl-6-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human A2B receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50159502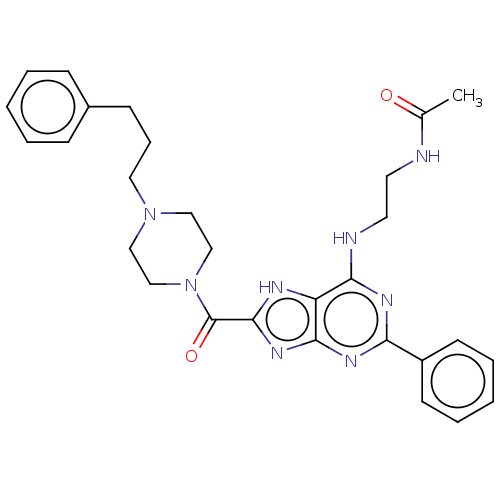 (CHEMBL3786849) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]OSIP339391 from human recombinant Adenosine A2B receptor expressed in HEK293 cells after 60 mins | J Med Chem 59: 1967-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01586 BindingDB Entry DOI: 10.7270/Q2QF8VS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408512 (US10358435, Example 29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408575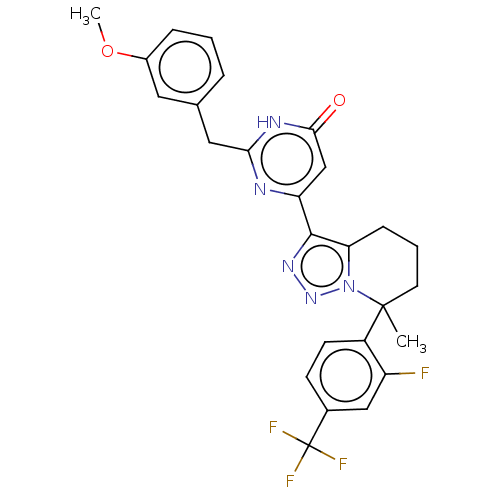 ((S)- or (R)-6-(7-(2-Fluoro-4- (trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM408512 (US10358435, Example 29) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM408573 (US10358435, Example 89 | US10358435, Example 90) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50576278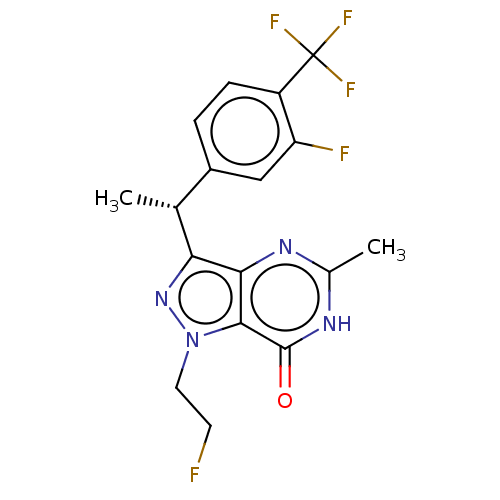 (CHEMBL4863002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE2 | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128082 BindingDB Entry DOI: 10.7270/Q2KK9GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408564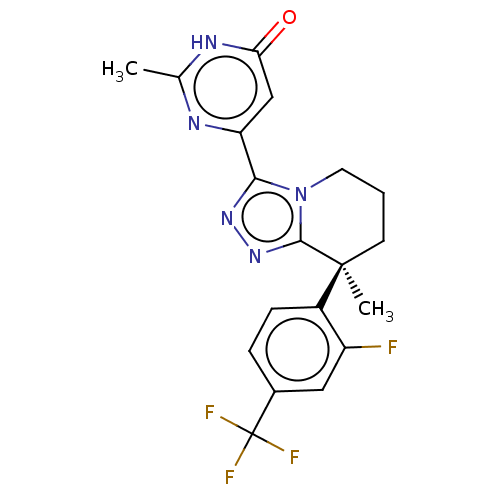 (US10358435, Example 80 | US10358435, Example 81) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50584560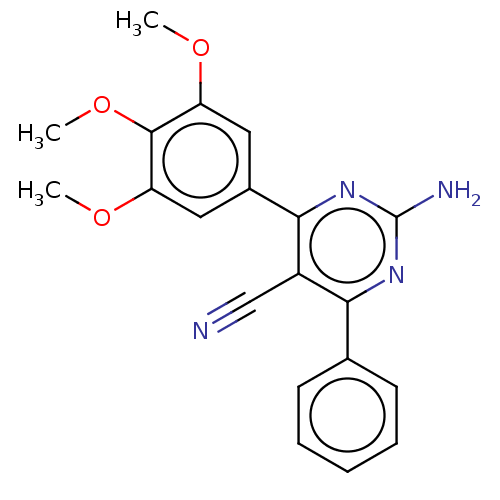 (CHEMBL5077370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50207816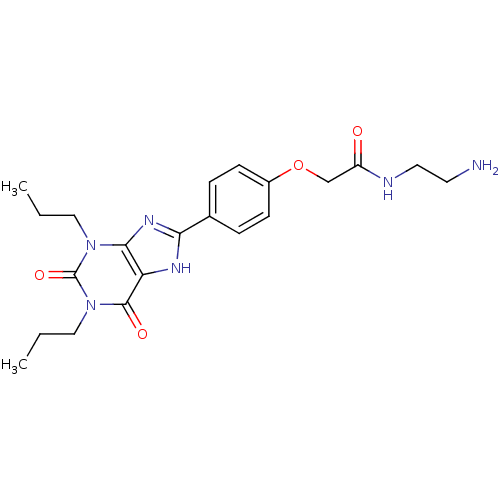 (CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50576275 (CHEMBL4873599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE2 | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128082 BindingDB Entry DOI: 10.7270/Q2KK9GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50161342 (2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]SCH-58261 from A2A adenosine receptor (unknown origin) expressed in HEK cell membrane incubated for 60 mins at room temperature b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375499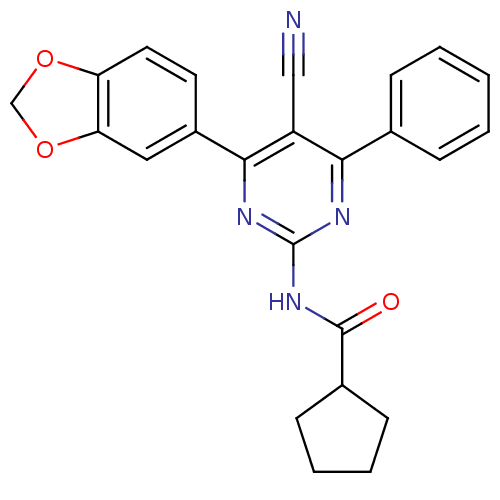 (CHEMBL259319) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from A1 adenosine receptor (unknown origin) expressed in CHO cell membrane incubated for 60 mins at room temperature by NXT... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408571 (US10358435, Example 87 | US10358435, Example 88) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50576279 (CHEMBL4871796) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE2 | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128082 BindingDB Entry DOI: 10.7270/Q2KK9GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408542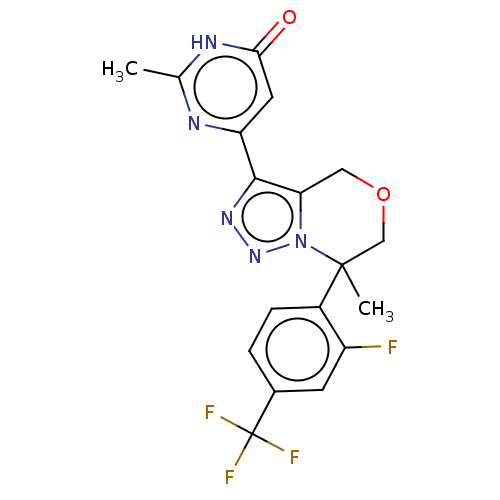 ((R)- or (S)-6-(7-(2-fluoro-4- (trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM408582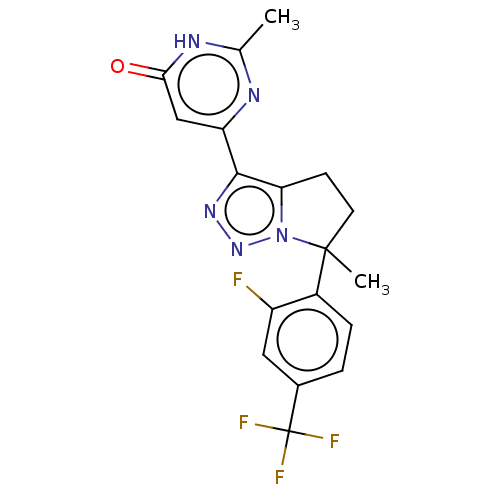 ((R)- or (S)-6-(6-(2-Fluoro-4- (trifluoromethyl)phe...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50584555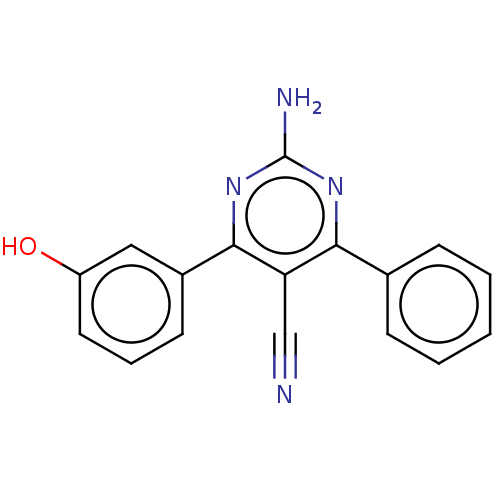 (CHEMBL5084351) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408582 ((R)- or (S)-6-(6-(2-Fluoro-4- (trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408515 (6-(1-(2-(2-chloro-4-ethylphenyl) propan-2-yl)-1H-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50576271 (CHEMBL4877091) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE2 | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128082 BindingDB Entry DOI: 10.7270/Q2KK9GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408518 (US10358435, Example 35 | US10358435, Example 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408513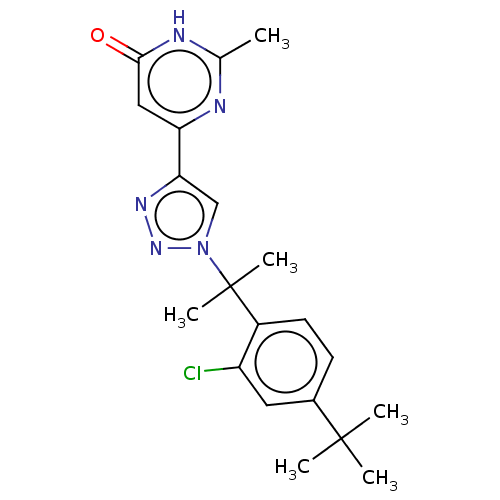 (6-(1-(2-(2-chloro-4-(trifluoromethyl) phenyl)propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM408564 (US10358435, Example 80 | US10358435, Example 81) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50584559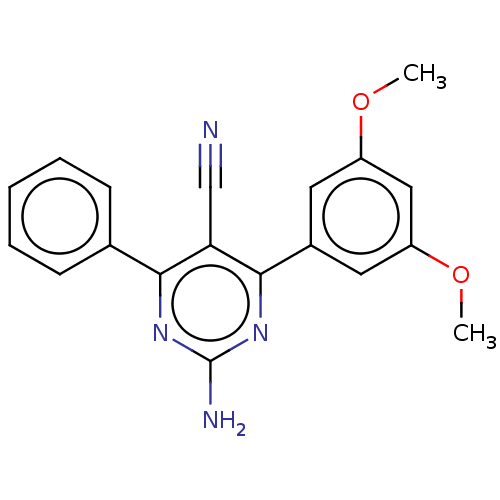 (CHEMBL5088876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408580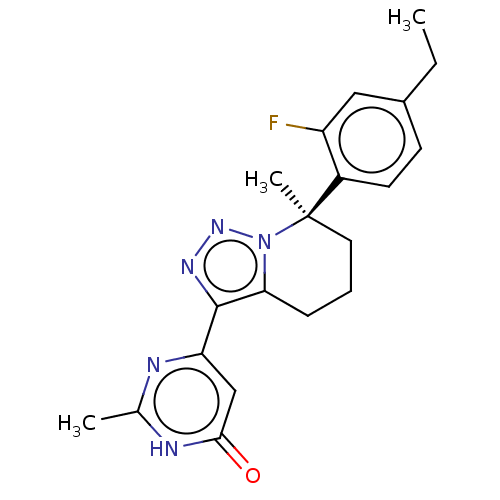 (US10358435, Example 95 | US10358435, Example 96) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375500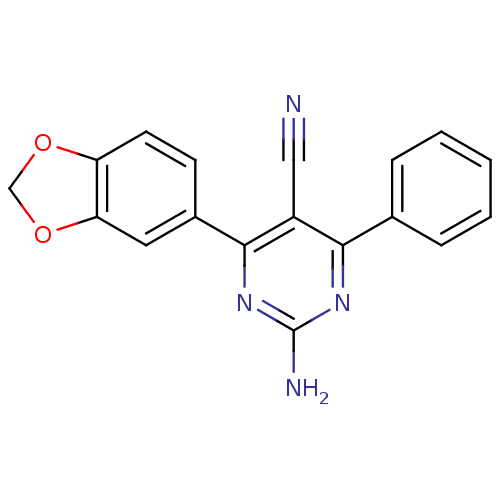 (CHEMBL259607) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM408571 (US10358435, Example 87 | US10358435, Example 88) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM385964 (US10287293, Example 13 | US10287293, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE2 | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128082 BindingDB Entry DOI: 10.7270/Q2KK9GNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human Adenosine A2A receptor expressed in HeLa cells after 30 mins | J Med Chem 59: 1967-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01586 BindingDB Entry DOI: 10.7270/Q2QF8VS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ZM2421385 from adenosine A2A receptor expressed in human HeLa cell membranes incubated for 30 mins by scintillation counting meth... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170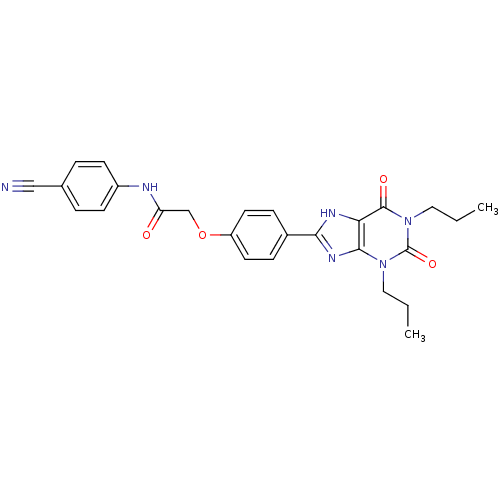 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [125I]IABOPX from human recombinant Adenosine A2B receptor expressed in HEK293 cells after 3 hrs | J Med Chem 59: 1967-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01586 BindingDB Entry DOI: 10.7270/Q2QF8VS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human A2B receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50089636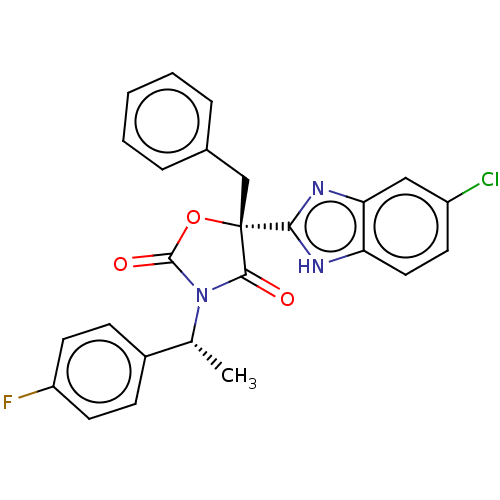 (CHEMBL3578271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor (unknown origin) | ACS Med Chem Lett 6: 461-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00010 BindingDB Entry DOI: 10.7270/Q21J9CH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408498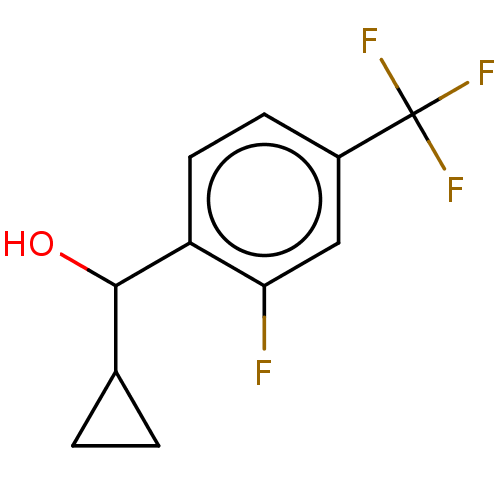 (US10358435, Example 19 | cyclopropyl(2-fluoro-4- (...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50584556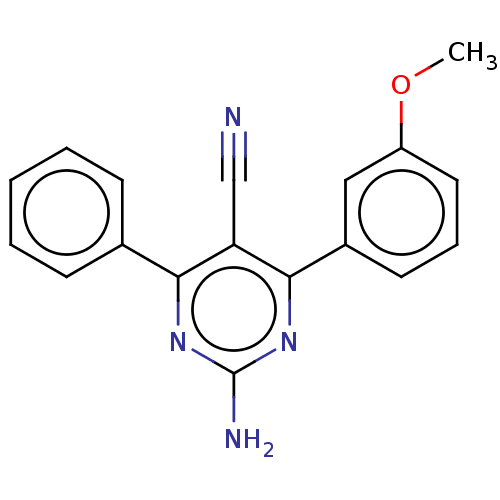 (CHEMBL5086406) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM408515 (6-(1-(2-(2-chloro-4-ethylphenyl) propan-2-yl)-1H-1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50584608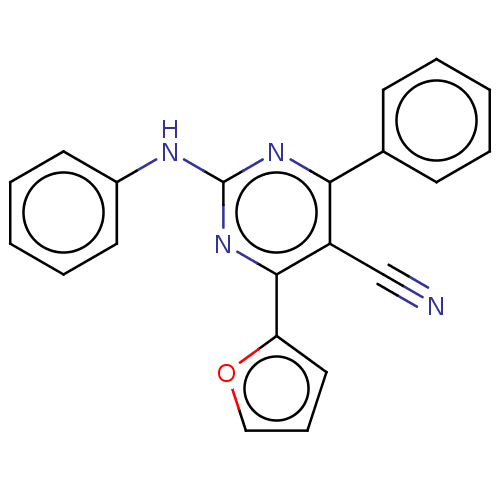 (CHEMBL5071174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human Adenosine A1 receptor expressed in CHO cells after 60 mins | J Med Chem 59: 1967-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01586 BindingDB Entry DOI: 10.7270/Q2QF8VS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes incubated for 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50584557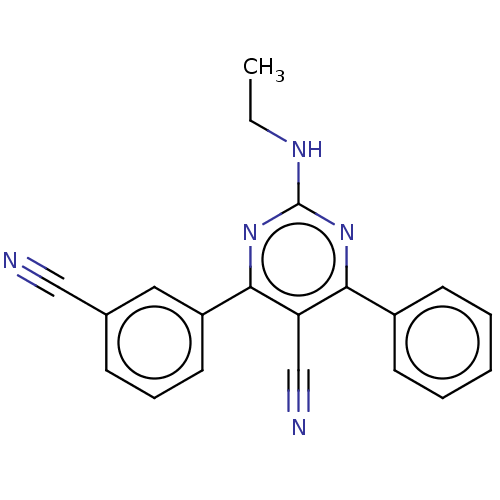 (CHEMBL5086487) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50584558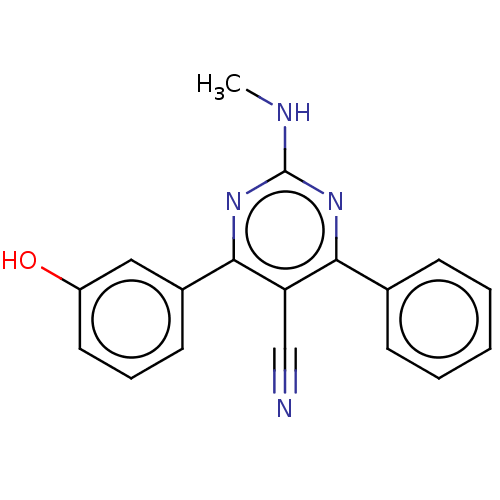 (CHEMBL5072557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50584560 (CHEMBL5077370) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50584559 (CHEMBL5088876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1AR expressed in CHO cell membrane incubated for 60 mins by microbeta trilux scintillation counter analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01636 BindingDB Entry DOI: 10.7270/Q2M61Q5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408500 ((R)- or (S)-6-(1-(1-(2,3-difluoro- 4-(trifluoromet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1343 total ) | Next | Last >> |