 Found 97 hits with Last Name = 'entrena' and Initial = 'a'
Found 97 hits with Last Name = 'entrena' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of arachidonic acid induced TXB2 generation in isolated human platelets (Prostaglandin G/H synthase 1 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325646
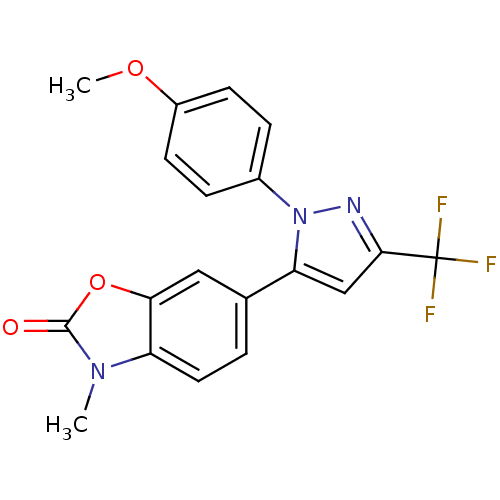
(1-(4-Methoxyphenyl)-3-trifluoromethyl-5-(3-methyl-...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc2n(C)c(=O)oc2c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-24-14-8-3-11(9-16(14)28-18(24)26)15-10-17(19(20,21)22)23-25(15)12-4-6-13(27-2)7-5-12/h3-10H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325646

(1-(4-Methoxyphenyl)-3-trifluoromethyl-5-(3-methyl-...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc2n(C)c(=O)oc2c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-24-14-8-3-11(9-16(14)28-18(24)26)15-10-17(19(20,21)22)23-25(15)12-4-6-13(27-2)7-5-12/h3-10H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325644
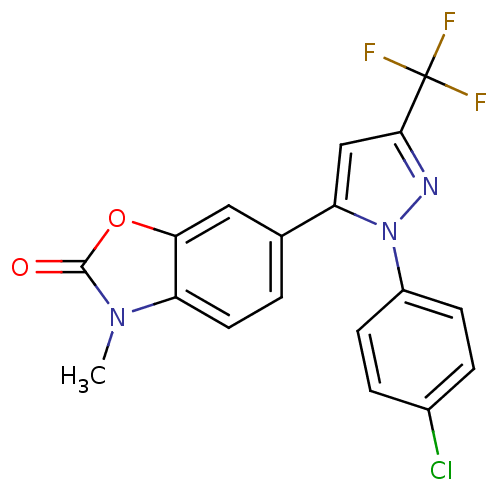
(1-(4-Chlorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C18H11ClF3N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325645
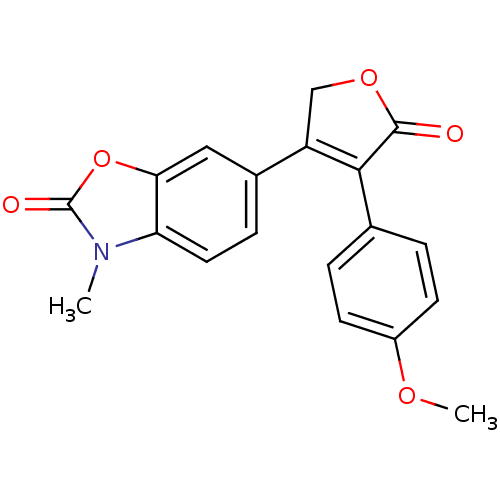
(3-(4-Methoxyphenyl)-4-(3-methyl-2-oxo-3H-benzoxazo...)Show SMILES COc1ccc(cc1)C1=C(COC1=O)c1ccc2n(C)c(=O)oc2c1 |t:9| Show InChI InChI=1S/C19H15NO5/c1-20-15-8-5-12(9-16(15)25-19(20)22)14-10-24-18(21)17(14)11-3-6-13(23-2)7-4-11/h3-9H,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 1 activity was evaluated in human whole blood as TXB2 production |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325645

(3-(4-Methoxyphenyl)-4-(3-methyl-2-oxo-3H-benzoxazo...)Show SMILES COc1ccc(cc1)C1=C(COC1=O)c1ccc2n(C)c(=O)oc2c1 |t:9| Show InChI InChI=1S/C19H15NO5/c1-20-15-8-5-12(9-16(15)25-19(20)22)14-10-24-18(21)17(14)11-3-6-13(23-2)7-4-11/h3-9H,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 2 activity was evaluated in human whole blood as LPS-induced PGE-2 generation |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110927
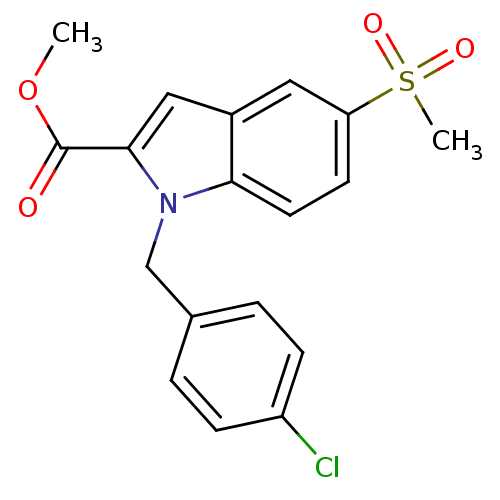
(1-(4-Chloro-benzyl)-5-methanesulfonyl-1H-indole-2-...)Show SMILES COC(=O)c1cc2cc(ccc2n1Cc1ccc(Cl)cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16ClNO4S/c1-24-18(21)17-10-13-9-15(25(2,22)23)7-8-16(13)20(17)11-12-3-5-14(19)6-4-12/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110930
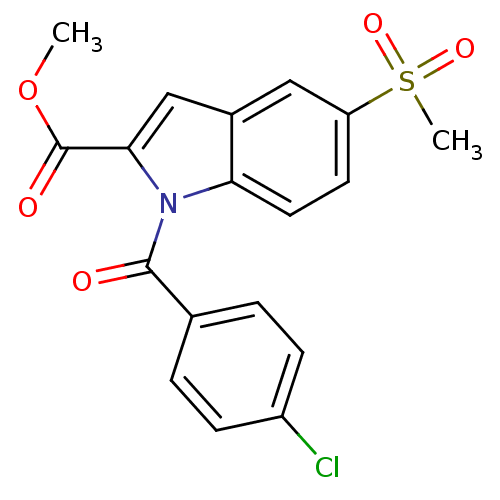
(1-(4-Chloro-benzoyl)-5-methanesulfonyl-1H-indole-2...)Show SMILES COC(=O)c1cc2cc(ccc2n1C(=O)c1ccc(Cl)cc1)S(C)(=O)=O Show InChI InChI=1S/C18H14ClNO5S/c1-25-18(22)16-10-12-9-14(26(2,23)24)7-8-15(12)20(16)17(21)11-3-5-13(19)6-4-11/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 2 activity was evaluated in human whole blood as LPS-induced PGE-2 generation |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110926

(5-Methanesulfonyl-1-(4-methoxy-benzyl)-1H-indole-2...)Show SMILES COC(=O)c1cc2cc(ccc2n1Cc1ccc(OC)cc1)S(C)(=O)=O Show InChI InChI=1S/C19H19NO5S/c1-24-15-6-4-13(5-7-15)12-20-17-9-8-16(26(3,22)23)10-14(17)11-18(20)19(21)25-2/h4-11H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325643

(1-(4-Fluorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H11F4N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325644

(1-(4-Chlorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C18H11ClF3N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of arachidonic acid induced TXB2 generation in isolated human platelets (Prostaglandin G/H synthase 1 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50279625
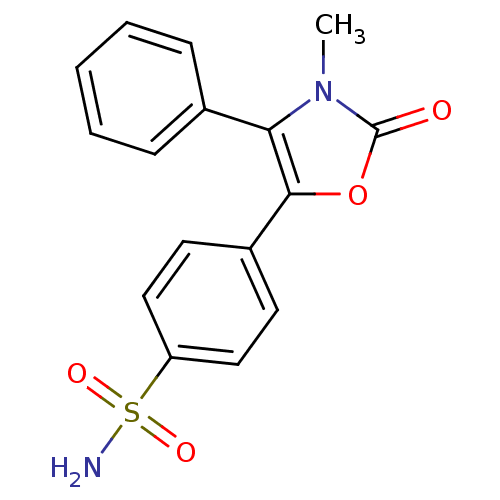
(4-(4-Phenyl-3-methyl-2-oxo-3H-1,3-oxazol-5-yl)benz...)Show SMILES Cn1c(c(oc1=O)-c1ccc(cc1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C16H14N2O4S/c1-18-14(11-5-3-2-4-6-11)15(22-16(18)19)12-7-9-13(10-8-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 2 activity was evaluated in human whole blood as LPS-induced PGE-2 generation |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 2 activity was evaluated in human whole blood as LPS-induced PGE-2 generation |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110923
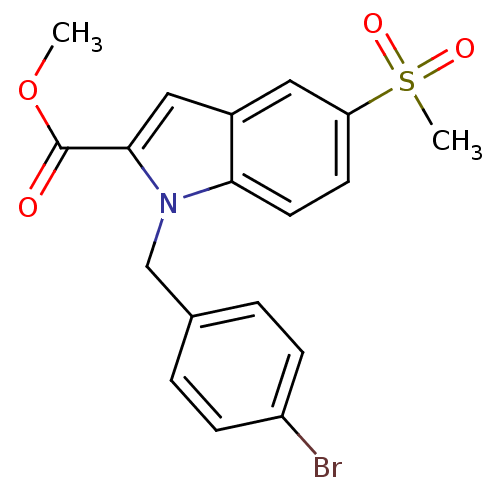
(1-(4-Bromo-benzyl)-5-methanesulfonyl-1H-indole-2-c...)Show SMILES COC(=O)c1cc2cc(ccc2n1Cc1ccc(Br)cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16BrNO4S/c1-24-18(21)17-10-13-9-15(25(2,22)23)7-8-16(13)20(17)11-12-3-5-14(19)6-4-12/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110927

(1-(4-Chloro-benzyl)-5-methanesulfonyl-1H-indole-2-...)Show SMILES COC(=O)c1cc2cc(ccc2n1Cc1ccc(Cl)cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16ClNO4S/c1-24-18(21)17-10-13-9-15(25(2,22)23)7-8-16(13)20(17)11-12-3-5-14(19)6-4-12/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 2 activity was evaluated in human whole blood as LPS-induced PGE-2 generation |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325643

(1-(4-Fluorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H11F4N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of arachidonic acid induced TXB2 generation in isolated human platelets (Prostaglandin G/H synthase 1 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110929

(1-(4-Bromo-benzoyl)-5-methanesulfonyl-1H-indole-2-...)Show SMILES COC(=O)c1cc2cc(ccc2n1C(=O)c1ccc(Br)cc1)S(C)(=O)=O Show InChI InChI=1S/C18H14BrNO5S/c1-25-18(22)16-10-12-9-14(26(2,23)24)7-8-15(12)20(16)17(21)11-3-5-13(19)6-4-11/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110924
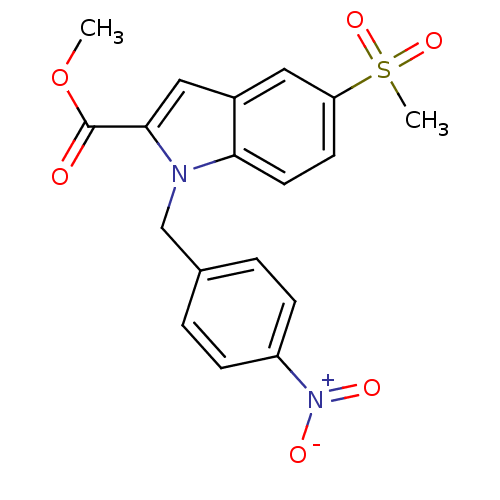
(5-Methanesulfonyl-1-(4-nitro-benzyl)-1H-indole-2-c...)Show SMILES COC(=O)c1cc2cc(ccc2n1Cc1ccc(cc1)[N+]([O-])=O)S(C)(=O)=O Show InChI InChI=1S/C18H16N2O6S/c1-26-18(21)17-10-13-9-15(27(2,24)25)7-8-16(13)19(17)11-12-3-5-14(6-4-12)20(22)23/h3-10H,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110925
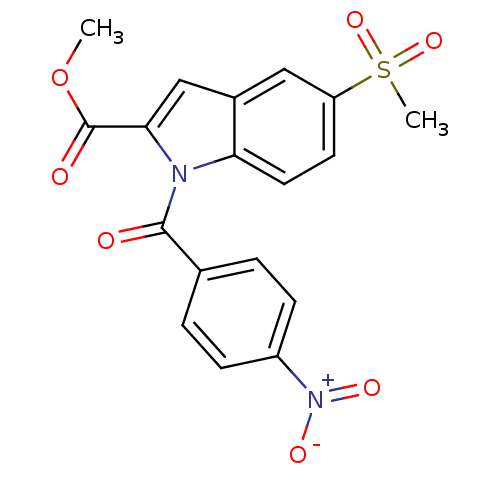
(5-Methanesulfonyl-1-(4-nitro-benzoyl)-1H-indole-2-...)Show SMILES COC(=O)c1cc2cc(ccc2n1C(=O)c1ccc(cc1)[N+]([O-])=O)S(C)(=O)=O Show InChI InChI=1S/C18H14N2O7S/c1-27-18(22)16-10-12-9-14(28(2,25)26)7-8-15(12)19(16)17(21)11-3-5-13(6-4-11)20(23)24/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110928

(1-(4-Chloro-benzyl)-5-methanesulfonyl-1H-indole-2-...)Show SMILES CS(=O)(=O)c1ccc2n(Cc3ccc(Cl)cc3)c(cc2c1)C(O)=O Show InChI InChI=1S/C17H14ClNO4S/c1-24(22,23)14-6-7-15-12(8-14)9-16(17(20)21)19(15)10-11-2-4-13(18)5-3-11/h2-9H,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibition of PGE-2 generation in LPS-stimulated human monocytes (Prostaglandin G/H synthase 2 cell assay) |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365106
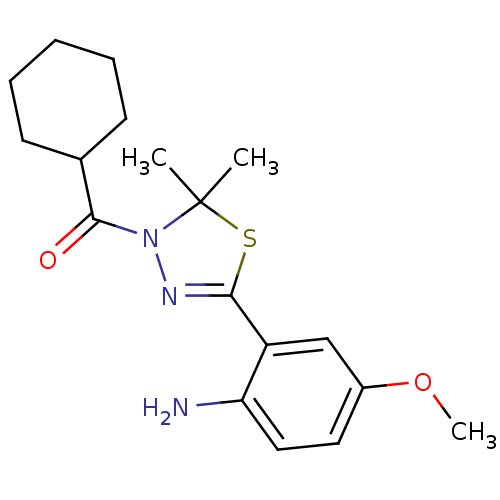
(CHEMBL1951503)Show SMILES COc1ccc(N)c(c1)C1=NN(C(=O)C2CCCCC2)C(C)(C)S1 |t:10| Show InChI InChI=1S/C18H25N3O2S/c1-18(2)21(17(22)12-7-5-4-6-8-12)20-16(24-18)14-11-13(23-3)9-10-15(14)19/h9-12H,4-8,19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 1 activity was evaluated in human whole blood as TXB2 production |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 1 activity was evaluated in human whole blood as TXB2 production |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365104
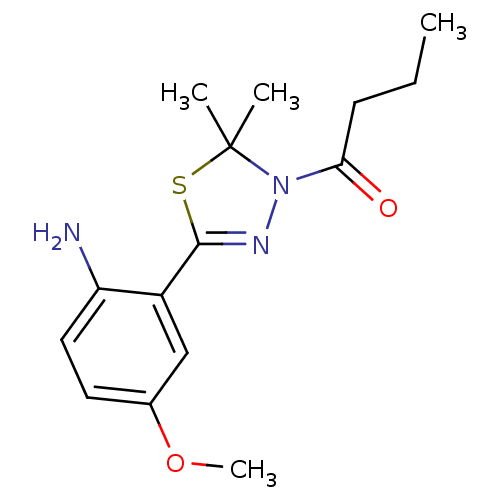
(CHEMBL1951498)Show InChI InChI=1S/C15H21N3O2S/c1-5-6-13(19)18-15(2,3)21-14(17-18)11-9-10(20-4)7-8-12(11)16/h7-9H,5-6,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365107
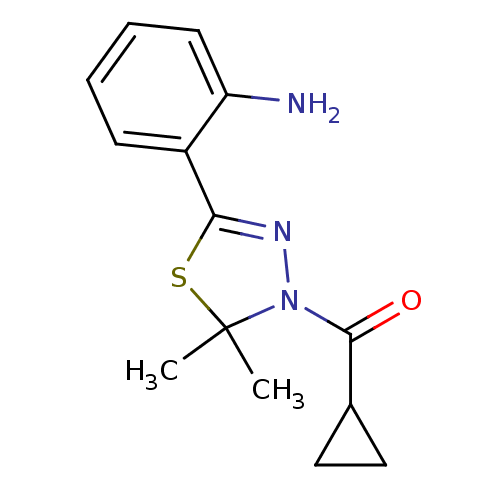
(CHEMBL1951517)Show InChI InChI=1S/C14H17N3OS/c1-14(2)17(13(18)9-7-8-9)16-12(19-14)10-5-3-4-6-11(10)15/h3-6,9H,7-8,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50108560

(2-Acetylamino-4-(2-amino-5-methoxy-phenyl)-4-oxo-b...)Show InChI InChI=1S/C13H16N2O5/c1-7(16)15-11(13(18)19)6-12(17)9-5-8(20-2)3-4-10(9)14/h3-5,11H,6,14H2,1-2H3,(H,15,16)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against Neuronal nitric oxide synthase (nNOS) activity |
J Med Chem 45: 263-74 (2002)
BindingDB Entry DOI: 10.7270/Q2474966 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50365105
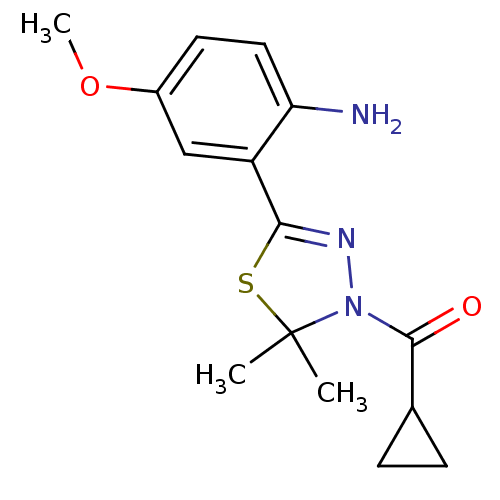
(CHEMBL1951500)Show SMILES COc1ccc(N)c(c1)C1=NN(C(=O)C2CC2)C(C)(C)S1 |t:10| Show InChI InChI=1S/C15H19N3O2S/c1-15(2)18(14(19)9-4-5-9)17-13(21-15)11-8-10(20-3)6-7-12(11)16/h6-9H,4-5,16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of iNOS assessed as conversion of L-[3H]arginine to L-[3H]-citrulline after 30 mins by scintillation counting |
Eur J Med Chem 50: 129-39 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.047
BindingDB Entry DOI: 10.7270/Q2V40VP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110926

(5-Methanesulfonyl-1-(4-methoxy-benzyl)-1H-indole-2...)Show SMILES COC(=O)c1cc2cc(ccc2n1Cc1ccc(OC)cc1)S(C)(=O)=O Show InChI InChI=1S/C19H19NO5S/c1-24-15-6-4-13(5-7-15)12-20-17-9-8-16(26(3,22)23)10-14(17)11-18(20)19(21)25-2/h4-11H,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Menarini S.A.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Prostaglandin G/H synthase 2 activity was evaluated in human whole blood as LPS-induced PGE-2 generation |
J Med Chem 45: 1402-11 (2002)
BindingDB Entry DOI: 10.7270/Q29G5M4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50279625

(4-(4-Phenyl-3-methyl-2-oxo-3H-1,3-oxazol-5-yl)benz...)Show SMILES Cn1c(c(oc1=O)-c1ccc(cc1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C16H14N2O4S/c1-18-14(11-5-3-2-4-6-11)15(22-16(18)19)12-7-9-13(10-8-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50492294
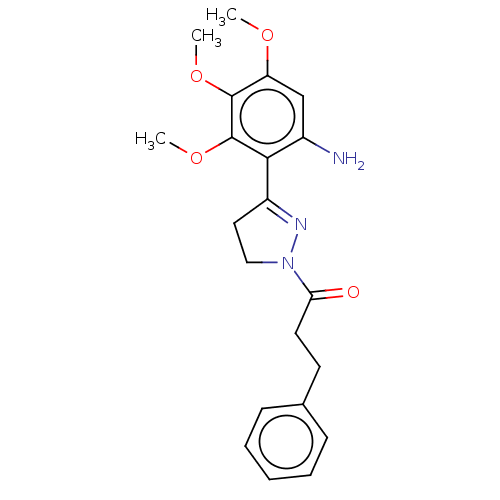
(CHEMBL2397627)Show SMILES COc1cc(N)c(C2=NN(CC2)C(=O)CCc2ccccc2)c(OC)c1OC |t:7| Show InChI InChI=1S/C21H25N3O4/c1-26-17-13-15(22)19(21(28-3)20(17)27-2)16-11-12-24(23-16)18(25)10-9-14-7-5-4-6-8-14/h4-8,13H,9-12,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of recombinant nNOS (unknown origin) assessed as conversion of L-[3H]-arginine to L-[3H]-citrulline after 30 mins |
Bioorg Med Chem 21: 4132-42 (2013)
Article DOI: 10.1016/j.bmc.2013.05.016
BindingDB Entry DOI: 10.7270/Q2CV4MPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50492295
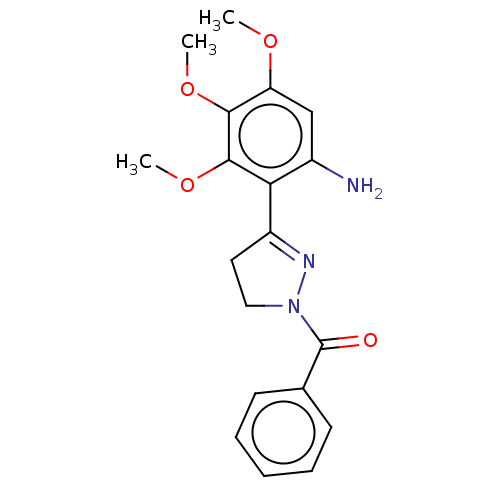
(CHEMBL2397625)Show SMILES COc1cc(N)c(C2=NN(CC2)C(=O)c2ccccc2)c(OC)c1OC |t:7| Show InChI InChI=1S/C19H21N3O4/c1-24-15-11-13(20)16(18(26-3)17(15)25-2)14-9-10-22(21-14)19(23)12-7-5-4-6-8-12/h4-8,11H,9-10,20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of recombinant nNOS (unknown origin) assessed as conversion of L-[3H]-arginine to L-[3H]-citrulline after 30 mins |
Bioorg Med Chem 21: 4132-42 (2013)
Article DOI: 10.1016/j.bmc.2013.05.016
BindingDB Entry DOI: 10.7270/Q2CV4MPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50492296
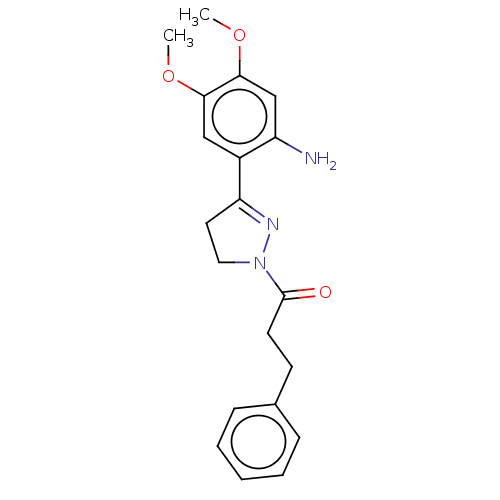
(CHEMBL2397623)Show SMILES COc1cc(N)c(cc1OC)C1=NN(CC1)C(=O)CCc1ccccc1 |t:12| Show InChI InChI=1S/C20H23N3O3/c1-25-18-12-15(16(21)13-19(18)26-2)17-10-11-23(22-17)20(24)9-8-14-6-4-3-5-7-14/h3-7,12-13H,8-11,21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of recombinant nNOS (unknown origin) assessed as conversion of L-[3H]-arginine to L-[3H]-citrulline after 30 mins |
Bioorg Med Chem 21: 4132-42 (2013)
Article DOI: 10.1016/j.bmc.2013.05.016
BindingDB Entry DOI: 10.7270/Q2CV4MPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50108561

(4-(2-Amino-5-methoxy-phenyl)-2-butyrylamino-4-oxo-...)Show InChI InChI=1S/C15H20N2O5/c1-3-4-14(19)17-12(15(20)21)8-13(18)10-7-9(22-2)5-6-11(10)16/h5-7,12H,3-4,8,16H2,1-2H3,(H,17,19)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against Neuronal nitric oxide synthase (nNOS) activity |
J Med Chem 45: 263-74 (2002)
BindingDB Entry DOI: 10.7270/Q2474966 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675
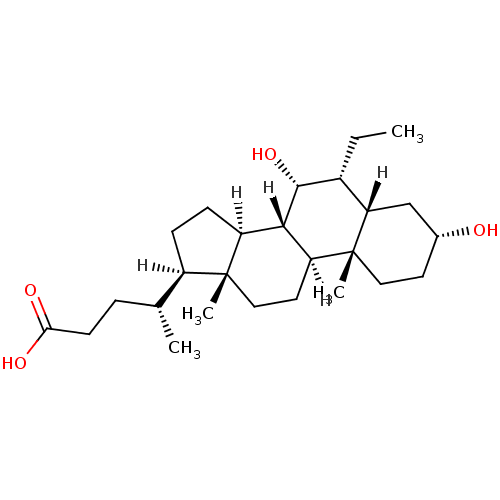
((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Binding affinity for human Farnesoid X receptor in FRET assay |
J Med Chem 47: 4559-69 (2004)
Article DOI: 10.1021/jm049904b
BindingDB Entry DOI: 10.7270/Q2JM2BD2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50451539
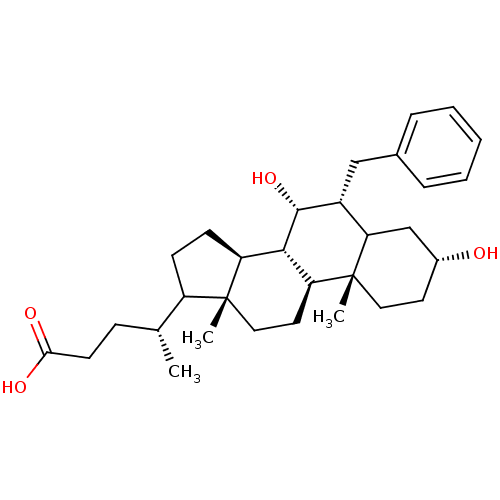
(CHEMBL3137828)Show SMILES [H][C@@]12CCC([C@H](C)CCC(O)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)[C@H](Cc2ccccc2)C2C[C@H](O)CC[C@]12C Show InChI InChI=1S/C31H46O4/c1-19(9-12-27(33)34)23-10-11-24-28-25(14-16-30(23,24)2)31(3)15-13-21(32)18-26(31)22(29(28)35)17-20-7-5-4-6-8-20/h4-8,19,21-26,28-29,32,35H,9-18H2,1-3H3,(H,33,34)/t19-,21-,22-,23?,24+,25+,26?,28+,29-,30-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Binding affinity for Farnesoid X Receptor (FXR) |
Bioorg Med Chem Lett 13: 1865-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Q52Q5J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data